Previous studies using blocking antibodies suggested that bone marrow (BM)–derived C3 is required for efficient osteoclast (OC) differentiation, and that C3 receptors are involved in this process. However, the detailed underlying mechanism and the possible involvement of other complement receptors remain unclear. In this report, we found that C3−/− BM cells exhibited lower RANKL/OPG expression ratios, produced smaller amounts of macrophage colony-stimulating factor and interleukin-6 (IL-6), and generated significantly fewer OCs than wild-type (WT) BM cells. During differentiation, in addition to C3, WT BM cells locally produced all other complement components required to activate C3 and to generate C3a/C5a through the alter-native pathway, which is required for efficient OC differentiation. Abrogating C3aR/C5aR activity either genetically or pharmaceutically suppressed OC generation, while stimulating WT or C3−/− BM cells with exogenous C3a and/or C5a augmented OC differentiation. Furthermore, supplementation with IL-6 rescued OC generation from C3−/− BM cells, and neutralizing antibodies to IL-6 abolished the stimulatory effects of C3a/C5a on OC differentiation. These data indicate that during OC differentiation, BM cells locally produce components, which are activated through the alternative pathway to regulate OC differentiation. In addition to C3 receptors, C3aR/C5aR also regulate OC differentiation, at least in part, by modulating local IL-6 production.
Introduction
C3 is the central part of the complement system, which is pivotal in fighting infection and clearing out immune complexes as part of the innate immunity. For C3 to impact any cell, it needs to be activated through 1 of the 3 activating pathways: the classical, the alternative, or the lectin pathway. To form the C3 convertase (C3bBb) that is required in the alternative-pathway complement-activation cascade, C3b is generated from spontaneous C3 hydrolysis and binds to the active form of factor B (Bb), which is produced by the enzymatic activity of factor D. After the enzymatic activities of these C3/C5 convertases, the resultant complement-activation products bind to their receptors, facilitating cell migration,1 phagocytosis,2 as well as many other cellular activities. For cell surface–bound complement activation products, such as C3b and its derivatives, there are the C3 receptors, CR1,3 CR2,4 CR3,5 and CR46 ; for the released complement-activation products, such as C3a and C5a,1 there are C3aR7 and C5aR.8 C3aR and C5aR are G protein-coupled receptors that are present on a broad spectrum of cells.9 C3a and C5a (also known as, anaphylatoxins) are small polypeptides,10 which bind to C3aR and C5aR with high affinity (Kd = 1 nM) to modulate many cell activities, including stimulating the production of interleukin (IL)-6 (reviewed in Haas and van Strijp11 ).
In 1993, Dr Suda and his colleagues reported that the activated form of vitamin D, 1,25(OH)2 vitamin D3, induces bone marrow (BM) cells to locally produce C3, and that blocking C3 or C3 receptors using respective monoclonal antibodies (mAbs) in BM cell cultures significantly inhibits 1,25(OH)2 vitamin D3–stimulated osteoclast (OC) differentiation.12 However, despite these intriguing results, how the locally generated C3 is activated, and whether C3aR or C5aR are involved in the process of osteoclast differentiation, and if so, by which mechanism, remains unclear.
In this report, using knockout mice deficient of C3, factor D, C3aR, and/or C5aR, we studied the role of complement in 1,25(OH)2 vitamin D3–induced OC differentiation. We found that, consistent with the previous report using C3-blocking mAbs,12 BM cells from C3−/− mice generated significantly decreased numbers of OC after stimulation. In accordance with these results, C3−/− BM cells exhibited reduced receptor activator of nuclear factor κB ligand (RANKL)/osteoprotegerin (OPG) expression ratios and produced decreased amounts of macrophage colony-stimulating factor (M-CSF) and IL-6 during OC differentiation. More importantly, we also found that in addition to C3, BM cells locally produce factor B, factor D, and C5 after 1,25(OH)2 vitamin D3 stimulation, and that the alternative pathway of complement activation is required to activate C3 for efficient OC differentiation. In addition to the C3 receptors reported before,12 our data suggest that C3aR/ C5aR are also integrally involved in OC differentiation, and their regulatory roles are mediated, at least in part, through modulating local IL-6 production.
Methods
Genetically engineered mice
Wild-type (WT) C57BL/6 and C3−/− mice13 were ordered from The Jackson Laboratory. Factor D−/− mice were gifts from Dr Yuanyuan Ma (University of Alabama at Birmingham),14 and factor B−/− mice15 were kindly provided by Dr Michael Holers (University of Colorado at Denver). C3aR−/−16 and C5aR−/−17 mice were generously provided by Dr Craig Gerard (Harvard University), and C3aR−/−C5aR−/− mice were identified by polymerase chain reaction (PCR) genotyping after crossing the C3aR−/− with C5aR−/− mice. All mice are on the C57BL/6 background, and all animal studies were performed under an approved protocol in accordance with the guidelines of the Institutional Animal Care and Use Committee of Case Western Reserve University.
BM-cell cultures
Human BM cells from healthy donors were obtained from the Hematopoietic Stem Cell Core Facility of Case Western Reserve University. Murine BM cells were isolated from 8- to 12-week-old female mouse femurs and tibias, washed, and collected in 15-mL tubes in α-modified Eagle medium (MEM) containing 10% fetal bovine serum (FBS) that was heat-inactivated to eliminate complement activity. For OC differentiation, 2 × 106 BM cells were cultured in complete α-MEM medium in wells of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3 (Cayman Chemical) as described before.12 Cultures were fed every 3 days with fresh media. For IL-6 or C5aR antagonist (C5aRA)/C3aR antagonist (C3aRA) supplementation experiments, 20 ng/mL IL-6 (R&D Systems) or 50μM of each antagonist or both (C5aRA: JPE-1375; custom synthesized by Anaspec; C3aRA:SB290157; purchased from BMD Chemicals) were included in the medium. For C3a and/or C5a supplementation experiments, 50 ng/mL purified C3a (BD Biosciences) or C5a (Cell Sciences) or both were added daily into WT and C3−/− BM-cell cultures. To neutralize IL-6 in samples treated with C3a/C5a, 10 ng/mL rat anti-IL-6 mAb (Clone 6B4 IGH 54; eBioscience) were added every 2 days. At day 12, differentiated OCs were identified by conventional tartrate-resistant acid phosphatase (TRAP) staining using a kit (Sigma-Aldrich) and following the protocol provided by the manufacturer. Total numbers of TRAP-positive cells in each well were counted under a microscope, and cells containing 3 or more nuclei were categorized as multinucleated.
Primary calvarial osteoblast and splenocyte cocultures
Primary calvarial osteoblasts (OBs) were isolated from newborn WT and C3−/− mice, following protocols described before.18 In brief, dissected calvariae from 2- to 3-day-old WT and C3−/− mice were digested with 1 mg/mL collagenase D (Roche) and 0.05% trypsin (Invitrogen) in Hanks buffered salt solution. Cells from second and third digestions were pooled and grown in α-MEM with antibiotics and 10% FBS. At confluence, cells were trypsinized and counted for coculture experiments. To set up coculture experiments, 2 × 104 primary calvarial OBs from WT or C3−/− mice were cultured with 2 × 106 splenocytes from WT and C3−/− mice together with 1 × 10−8 1,25(OH)2 vitamin D3. The resultant TRAP-positive cells were counted after 12 days of coculture.
Complement assays
To determine that functional factor B, factor D, and C5 are produced during OC differentiation, culture supernatants were collected at day 6 from 2 × 106 WT BM cells stimulated with 1 × 10−8M 1,25(OH)2 vitamin D3 and assayed for complement activities. For functional factor B and D detection, collected culture supernatants were directly added into zymosan C3 uptake assays with factor B or D deficient mouse sera. In brief, sera were prepared by bleeding male WT, factor B−/−, or factor D−/− mice through the tail vein. After this, 20% BM cell-conditioned culture supernatants or control media were added into 10% of WT, factor B−/−, or factor D−/− serum together with 30 μg/mL zymosan (Sigma-Aldrich) in gelatin veronal buffer (GVB)/Mg2+-EGTA (ethylene glycol tetraacetic acid) buffer and incubated at 37°C for 30 minutes. After washing, C3b deposition was assessed by fluorescein isothiocyanate (FITC)–anti–mouse C3 mAb (Cedarlane) staining, followed by flow cytometry analysis. For functional C5 detection, BM cell-culture supernatant or control media was concentrated 10-fold using a Microcon (Millipore) and added into EshA-based hemolytic assays using 10% of human C5-depleted serum (Complement Tech). The ability of the BM cell–conditioned media to compensate the C5 deficiency in the serum was assessed by measuring OD541 to quantify C5b-9 (membrane attack complex)–mediated sheep erythrocyte hemolysis.
IL-6 and M-CSF ELISA
For IL-6–level measurements, culture supernatants were collected at day 12 after 1,25(OH)2 vitamin D3 stimulation, and standard IL-6 enzyme-linked immunosorbent assay (ELISA; R&D Systems) was used according to the protocol provided by the manufacturer. For M-CSF assays, culture supernatants were collected at day 3 after stimulation, concentrated by ultrafiltration using a centrifugal concentrator (molecular weight [MW] cutoff: 3000; Millipore), then analyzed by a murine M-CSF ELISA kit (PeproTech), following the manufacturer-provided protocol.
Assessment of RANKL/OPG expression ratios
To compare RANKL/OPG expression levels in WT and C3−/− BM cells during OC differentiation, 2 × 106 BM cells were isolated from WT or C3−/− mice and cultured in complete α-MEM with and without the presence of 1 × 10−8M 1,25(OH)2 vitamin D3. At 24 hours, total RNA was purified from the cells using TRIzol (Invitrogen), and reverse transcribed using a first-strand cDNA synthesis kit (Invitrogen), following protocols provided by the manufacturer. The relative expression levels of RANKL and OPG were assessed by SYBR Green-based quantitative reverse transcription (RT)–PCR (qRT-PCR; GoTag qPCR master mix; Promega). In brief, the qRT-PCR was carried out in triplicate for RANKL, OPG, and β-actin (internal control) of each sample on an ABI PRISM 7500 machine (Applied Biosystems). The data were analyzed and normalized against levels of RANKL and OPG in BM cells without 1,25(OH)2 vitamin D3 stimulation by the 7500 SDS Version 1.3 software package (Applied Biosystems). Dissociation experiments were used to ensure that the fluorescent signal for each amplicon was derived from the PCR products only.
Statistical analysis
All experiments were repeated at least twice. Results were compared using the Student t test. A P value < .05 was considered significant.
Results
C3−/− BM cells generate fewer OCs than WT BM cells
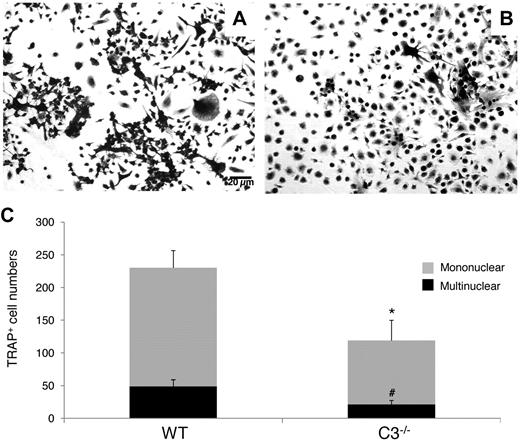
To examine whether the absence of locally produced C3 from BM cells will inhibit OC generation, as suggested by previous reports using C3 blocking mAbs,12 we incubated WT and C3−/− BM cells with 1,25(OH)2 vitamin D3, using the same protocol as in previous studies,12,19 and compared the number of TRAP-positive cells on day 12. As shown in Figure 1, after stimulation, C3−/− BM cells produced 119 ± 31 TRAP-positive mononuclear cells and 21 ± 6 multinucleated TRAP-positive cells per well, while WT BM cells generated 230 ± 26 TRAP-positive mononuclear cells and 49 ± 10 multinucleated TRAP-positive cells (P < .05). These results indicate that the absence of C3 from BM cells reduces 1,25(OH)2 vitamin D3-stimulated OC differentiation by nearly 50%.
C3 produced by BM cells is required for efficient OC differentiation. Aliquots of 2 × 106 WT and C3−/− BM cells were cultured in α-MEM/10% heat-inactivated FBS media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3. OCs were stained, and TRAP-positive cells in each well were counted under a microscope. Panels show representative TRAP-positive cells from WT (A), C3−/− (B), BM cells, and total mononuclear (mono)/multinucleated (multi) TRAP-positive cells in each well (C). Results are representative from 3 independent experiments performed in triplicate. Data are mean ± SD: *P < .05, #P < .05.
C3 produced by BM cells is required for efficient OC differentiation. Aliquots of 2 × 106 WT and C3−/− BM cells were cultured in α-MEM/10% heat-inactivated FBS media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3. OCs were stained, and TRAP-positive cells in each well were counted under a microscope. Panels show representative TRAP-positive cells from WT (A), C3−/− (B), BM cells, and total mononuclear (mono)/multinucleated (multi) TRAP-positive cells in each well (C). Results are representative from 3 independent experiments performed in triplicate. Data are mean ± SD: *P < .05, #P < .05.
C3−/− BM cells produced reduced levels of M-CSF and failed to efficiently up-regulate RANKL expression after 1,25(OH)2 vitamin D3 stimulation
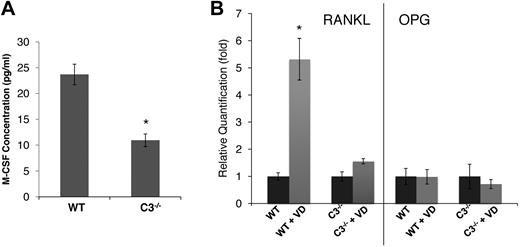
In light of previous findings on the critical role of M-CSF in the process of OC differentiation,20,21 we next assessed the levels of M-CSF in WT and C3−/− BM cell-culture supernatants using a standard ELISA kit. These assays showed that, compared with 23.7 ± 2.1 pg/mL M-CSF in WT BM cell-conditioned media, C3−/− BM cell–conditioned media contained only 10.9 ± 1.2 pg/mL of M-CSF (Figure 2A). Because of the well-established roles of RANKL22,23 and OPG24,25 in osteoclast generation, we also analyzed the expression levels of RANKL and OPG in WT and C3−/− BM cells after 1,25(OH)2 vitamin D3 stimulation by qRT-PCR. Consistent with previous reports by others,23,26 in WT BM cells, 1,25(OH)2 vitamin D3 stimulation markedly up-regulated RANKL expression (∼ 6-fold), but had little effect on OPG expression (Figure 2B). However, the same assays demonstrated that C3−/− BM cells failed to significantly up-regulate RANKL expression (1.6-fold), and that the expression levels of OPG remained essentially unchanged. These results indicate that during OC differentiation, C3−/− BM cells produced decreased levels of M-CSF and decreased RANKAL/OPG ratios, which is in accordance with the lower numbers of OCs generated.
C3-deficient BM cells produce decreased amounts of M-CSF and failed to up-regulate RANKL during differentiation. (A) M-CSF levels were measured by ELISA in WT and C3−/− BM cell-conditioned media during differentiation. (B) RANKL/OPG expression levels were quantified by qRT-PCR in WT and C3−/− BM cells after 1,25(OH)2 vitamin D3 (VD) stimulation. Results are representative from 2 independent experiments performed in triplicates. Data are mean ± SD: *P < .05.
C3-deficient BM cells produce decreased amounts of M-CSF and failed to up-regulate RANKL during differentiation. (A) M-CSF levels were measured by ELISA in WT and C3−/− BM cell-conditioned media during differentiation. (B) RANKL/OPG expression levels were quantified by qRT-PCR in WT and C3−/− BM cells after 1,25(OH)2 vitamin D3 (VD) stimulation. Results are representative from 2 independent experiments performed in triplicates. Data are mean ± SD: *P < .05.
BM cells locally produce functional factor B, factor D, and C5 in addition to C3 during differentiation
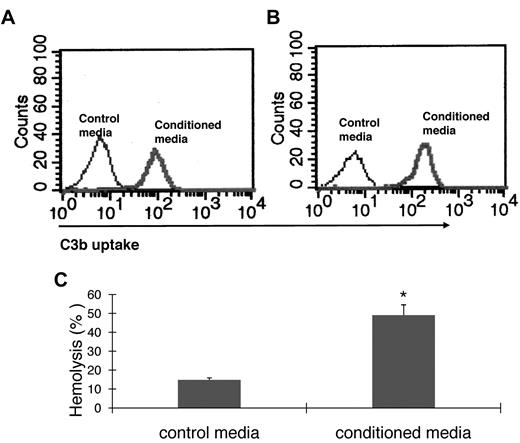
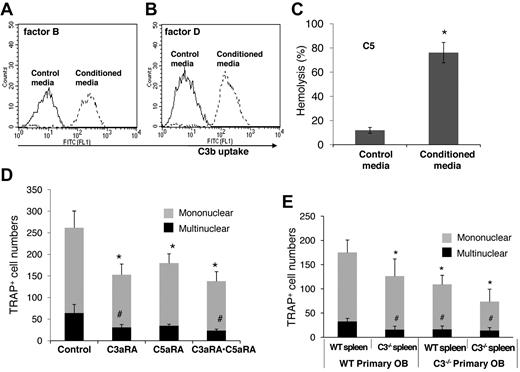
For C3 to impact any cell, it first needs to be activated. To examine how BM cell-generated C3 could be activated to regulate OC differentiation, we assessed the presence of factor B and factor D, components essential for the activation of C3 through the alternative pathway of complement activation, as well as C5, the component required for C5a generation. The presence of mRNA for factor B, factor D, and C5 were first confirmed by RT-PCR (data not shown), and then the presence of complement proteins were tested with functional assays of the BM cell-conditioned media using sera deficient of factor B, factor D, or C5. These assays (Figure 3) showed that after 1,25(OH)2 vitamin D3 stimulation, BM cell-conditioned media compensated the absence of factor B (Figure 3A), factor D (Figure 3B), or C5 (Figure 3C) in zymosan-based C3b uptake and EshA-based hemolytic assays, indicating that BM cells produce functional factor B, factor D, and C5 during differentiation. These results indicate that C3 could be activated through the alternative pathway during BM cell differentiation, leading to the production of complement activation products, including ligands for C3 receptors and the released anaphylatoxins, C3a/C5a.
BM cells locally produce functional factor B, factor D, and C5. (A) Zymosan-based C3b uptake assays using factor B−/− sera plus 1:5 diluted control media (thin line) or BM cell–conditioned media (thick line), showing that BM cell–conditioned media compensated the absence of factor B. (B) Zymosan-based C3b uptake assays using factor D−/− sera plus 1:5 diluted control media (thin line) or BM cell-conditioned media (thick line), showing that BM cell-conditioned media compensated the absence of factor D. (C) EshA-hemolytic assays using C5-depleted sera plus 1:5 diluted control media or BM cell-conditioned media, showing that BM cell-conditioned media compensated the absence of C5, therefore inducing C5b-9 mediated hemolysis. Representative results of > 4 independent experiments.
BM cells locally produce functional factor B, factor D, and C5. (A) Zymosan-based C3b uptake assays using factor B−/− sera plus 1:5 diluted control media (thin line) or BM cell–conditioned media (thick line), showing that BM cell–conditioned media compensated the absence of factor B. (B) Zymosan-based C3b uptake assays using factor D−/− sera plus 1:5 diluted control media (thin line) or BM cell-conditioned media (thick line), showing that BM cell-conditioned media compensated the absence of factor D. (C) EshA-hemolytic assays using C5-depleted sera plus 1:5 diluted control media or BM cell-conditioned media, showing that BM cell-conditioned media compensated the absence of C5, therefore inducing C5b-9 mediated hemolysis. Representative results of > 4 independent experiments.
Alternative pathway of complement activation is required for efficient OC differentiation
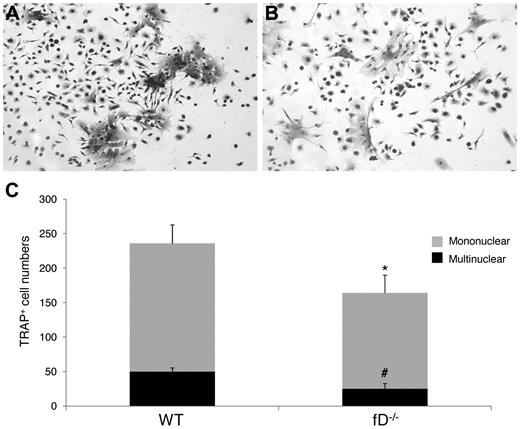
To determine whether the alternative pathway of complement activation is required to activate the C3 locally produced by the BM cells, and thus implicating complement activation in the regulation of OC differentiation, we compared the numbers of OCs generated from WT and factor D−/− BM cells after 1,25(OH)2 vitamin D3 stimulation. As described earlier, factor D is essential for the alternative pathway of complement activation. These osteoclast assays (Figure 4) showed that factor D−/− BM cells generated 163 ± 16 mononuclear and 25 ± 8 multinucleated TRAP-positive cells per well, compared with 236 ± 16 mononuclear and 50 ± 2 multinucleated TRAP-positive cells in wells containing WT BM cells (P < .05). These results indicate that the alternative pathway of complement activation is required to activate C3 for efficient OC differentiation from BM cells after 1,25(OH)2 vitamin D3 stimulation.
The alternative pathway of complement activation is required efficient OC differentiation. Aliquots of 2 × 106 WT and factor D−/− BM cells were cultured in α-MEM/10% heat-inactivated FBS media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3. OCs were stained, and TRAP-positive cells in each well were counted under a microscope. Panels show representative TRAP-positive cells from WT (A), factor D−/− (B) BM cells, and total mononuclear (mono)/multinucleated (multi) TRAP-positive cells in each well (C). Results are representative from 2 independent experiments performed in triplicates. Data are mean ± SD: *P < .05, #P < .05.
The alternative pathway of complement activation is required efficient OC differentiation. Aliquots of 2 × 106 WT and factor D−/− BM cells were cultured in α-MEM/10% heat-inactivated FBS media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3. OCs were stained, and TRAP-positive cells in each well were counted under a microscope. Panels show representative TRAP-positive cells from WT (A), factor D−/− (B) BM cells, and total mononuclear (mono)/multinucleated (multi) TRAP-positive cells in each well (C). Results are representative from 2 independent experiments performed in triplicates. Data are mean ± SD: *P < .05, #P < .05.
C3aR and C5aR are required for efficient OC differentiation
Since BM cells locally produce both C3 and C5 during OC differentiation, and their activation through the alternative pathway can generate C3a and C5a, we next examined whether the receptors for these ligands, C3aR and C5aR, could regulate OC differentiation. We isolated BM cells from WT, C3aR−/−, C5aR−/−, and C3aR−/−C5aR−/− mice, then incubated them with 1,25(OH)2vitamin D3, following the same OC differentiation protocol. The osteoclast induction assays (Figure 5A) demonstrated that, compared with WT BM cells (226 ± 22 mononuclear and 55 ± 5 multinucleated cells), C3aR−/− and C5aR−/− BM cells generated a reduced number of TRAP-positive cells; C3aR−/− BM cells had 115 ± 12 mononuclear and 20 ± 3 multinucleated cells, and C5aR−/− BM cells had 164 ± 6 mononuclear and 40 ±5 multinucleated cells, while the double-knockout C3aR−/−C5aR−/− BM cells had the least number of TRAP-positive cells (91 ±10 mononuclear and 20 ± 7 multinuclear cells). In complementary experiments, during 1,25(OH)2 vitamin D3 stimulation, we treated WT BM cells with C3aRA (SB290157), C5aRA (JPE-1375), or both. In these assays (Figure 5B), compared with the placebo (217 ±25 mononuclear and 42 ± 9 multinuclear cells), C3aRA significantly inhibited OC generation (156 ± 23 mononuclear and 29 ±7 multinuclear cells). While C5aRA appeared to reduce numbers of both mono- and multinucleated TRAP-positive cells (176 ± 29 mononuclear and 34 ± 8 multinuclear cells), it did not reach a statistical significance (P = .068 for mononuclear cells and P = .12 for multinucleated cells). However, a combination of C3aRA and C5aRA inhibited OC generation synergistically (66 ± 4 mononuclear and 16 ± 2 multinuclear cells; P < .05). These results indicate that in addition to C3 receptors, C3aR and, possibly, C5aR are also required for efficient OC generation, in which C3aR may play a more prominent role than C5aR.
C3aR/C5aR are required for efficient OC differentiation. (A) 2 × 106 WT,C3aR−/−,C5aR−/−, and C3aR−/−C5aR−/− BM cells were cultured in α-MEM/10% heat-inactivated media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3. OCs were stained, and TRAP-positive cells in each well were counted under a microscope. (B) WT BM cells (2 × 106) were cultured in α-MEM/10% heat-inactivated FBS media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3 in the presence of placebo (control), C3aRA, C5aRA, or C3aRA·C5aRA. OCs were stained, and TRAP-positive cells in each well were counted under a microscope. Results are representative from 2 independent experiments performed in triplicate. Data are mean ± SD *P < .05, #P < .05.
C3aR/C5aR are required for efficient OC differentiation. (A) 2 × 106 WT,C3aR−/−,C5aR−/−, and C3aR−/−C5aR−/− BM cells were cultured in α-MEM/10% heat-inactivated media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3. OCs were stained, and TRAP-positive cells in each well were counted under a microscope. (B) WT BM cells (2 × 106) were cultured in α-MEM/10% heat-inactivated FBS media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin D3 in the presence of placebo (control), C3aRA, C5aRA, or C3aRA·C5aRA. OCs were stained, and TRAP-positive cells in each well were counted under a microscope. Results are representative from 2 independent experiments performed in triplicate. Data are mean ± SD *P < .05, #P < .05.
C3aR/C5aR regulated IL-6 expression is involved in OC differentiation
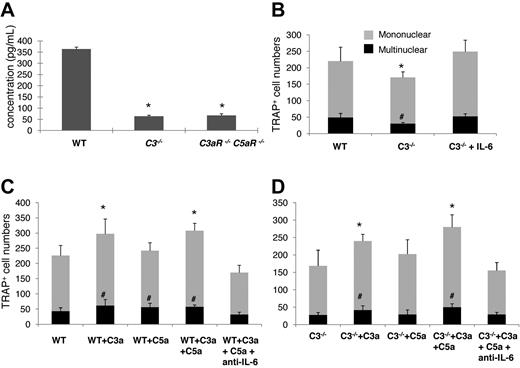
We next explored a potential mechanism underlying the C3aR/C5aR-mediated effects on OC differentiation, which is based on previous reports that IL-6 augments OC generation,27,28 and that C3aR/C5aR stimulates IL-6 production in many types of cells.11 We measured IL-6 levels by ELISA in WT, C3−/−, and C3aR−/−C5aR−/− BM cell-conditioned media after 1,25(OH)2 vitamin D3 stimulation. These measurements showed that, compared with 357.5 ± 66.4 pg/mL IL-6 in WT BM cell-conditioned media, there was only 72.8 ± 12.7 or 86.2 ± 16.3 pg/mL IL-6 in C3−/− or C3aR−/−C5aR−/− BM cell-conditioned media (Figure 6A).
Complement regulated IL-6 expression is involved in OC differentiation. (A) Quantification of IL-6 levels in supernatants cultured with WT, C3−/−, and C3aR−/−C5aR−/− BM cells during differentiation. BM cells (2 × 106) were cultured in differentiation media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin, and IL-6 levels were measured in supernatants on day 12. (B) Supplementing IL-6 into C3−/− BM cell cultures restored their OC differentiation capabilities. (C) Exogenous C3a/C5a augmented OC differentiation from WT BM cells, while neutralization of IL-6 abolished the stimulating effect. (D) Exogenous C3a/C5a augmented OC differentiation from C3−/− BM cells, while neutralization of IL-6 abolished the stimulating effect. Results are representative from 2 independent experiments performed in triplicate. Data are mean ± SD: *P < .05, #P < .05.
Complement regulated IL-6 expression is involved in OC differentiation. (A) Quantification of IL-6 levels in supernatants cultured with WT, C3−/−, and C3aR−/−C5aR−/− BM cells during differentiation. BM cells (2 × 106) were cultured in differentiation media in each well of a 24-well plate together with 1 × 10−8M 1,25(OH)2 vitamin, and IL-6 levels were measured in supernatants on day 12. (B) Supplementing IL-6 into C3−/− BM cell cultures restored their OC differentiation capabilities. (C) Exogenous C3a/C5a augmented OC differentiation from WT BM cells, while neutralization of IL-6 abolished the stimulating effect. (D) Exogenous C3a/C5a augmented OC differentiation from C3−/− BM cells, while neutralization of IL-6 abolished the stimulating effect. Results are representative from 2 independent experiments performed in triplicate. Data are mean ± SD: *P < .05, #P < .05.
To causally link reduced levels of IL-6 to decreased OC differentiation in C3−/− BM cells, we repeated the differentiation experiments with WT and C3−/− BM cells and, this time, supplemented 20 ng/mL of IL-6 into the C3−/− BM cell-culture daily and counted TRAP-positive cells at 12 days. As shown in Figure 6B, supplementation of exogenous IL-6 into C3−/− BM cell cultures increased the numbers of TRAP-positive cells from 108 ± 17 (mononuclear) and 31 ± 4 (multinucleated) to 249 ± 39 (mononuclear) and 52 ± 9 (multinucleated) (P < .05), suggesting that complement regulates OC differentiation, at least in part, through modulating local IL-6 production.
To further verify that C3aR/C5aR are integrally involved in OC differentiation, and IL-6 is the underlying mechanism, we next incubated WT or C3−/− BM cells with purified C3a, C5a, or both during the differentiation process, and neutralized IL-6 using an anti-IL-6 mAb in the cultures stimulated with C3a/C5a. These assays showed that while exogenous C3a increased the numbers of resultant OC from both WT and C3−/− BM cells, C5a did not appear to have a significant effect on TRAP+ cell formation (Figure 6C-D). Neutralizing IL-6 totally ablated the effects of C3a/C5a on augmenting OC generation from both the WT and C3−/− BM cells. Interestingly, exogenous C3a and the combination of C3a/C5a appeared to have a greater effect on C3−/− BM cells than WT BM cells (66.7% increase vs. 36.2% increase of mononuclear cells and 87.5% increase vs. 33.3% increase of multinucleated cells), possibly due to the lack of endogenous C3a/C5a production in C3−/− BM cells, while WT BM cells can still make the baseline of C3a/C5a during differentiation. Similarly, neutralization of IL-6 after C3a/C5a treatment in WT BM cells reduced OC numbers below placebo-treated WT BM cells, while neutralizing IL-6 in C3−/− BM cells just ablated C3a/C5a effects without further reducing OC numbers below the baseline, which is consistent with previous reports by others that IL-6 is critical in OC differentiation,27,28 and our findings that C3−/− BM cells only produce trace amount of IL-6 during OC differentiation.
Locally produced complement also regulates OC differentiation in humans
To determine whether the above-observed results would apply to humans, we subjected normal human BM cells to OC differentiation conditions with and without the C3aR/C5aR antagonists, then analyzed the conditioned media for the presence of factor B, factor D, and C5, and quantified the resultant TRAP+ cells. These assays showed that, like the results with the mouse system, human BM cells locally produce functional factor B (Figure 7A), factor D (Figure 7B), and C5 (Figure 7C) during OC differentiation, and that blocking C3aR and/or C5aR significantly inhibited OC generation (Figure 7D).
Complement is locally produced and required for efficient OC differentiation in humans. (A) Zymosan-based C3b uptake assays using factor B−/− sera plus 1:5 diluted control media (solid line) or human BM cell–conditioned media (dotted line), showing that BM cell-conditioned media compensated the absence of factor B. (B) Zymosan-based C3b uptake assays using factor D−/− sera plus 1:5 diluted control media (solid line) or human BM cell-conditioned media (dotted line), showing that BM cell-conditioned media compensated the absence of factor D. (C) EshA-hemolytic assays using C5-depleted sera plus 1:5 diluted control media or BM cell-conditioned media, showing that BM cell-conditioned media compensated the absence of C5, therefore inducing C5b-9–mediated hemolysis. (D) Human BM cells were incubated with 1 × 10−8M 1,25(OH)2 vitamin D3 in the presence of placebo (control), C3aRA, C5aRA, or C3aRA·C5aRA, showing that efficient OC differentiation in humans also requires C3aR/C5aR as in mice. Representative results of 2 independent experiments. Data are mean ± SD: *P < .05, #P < .05. (E) Both mesenchymal cells and OC progenitors are involved in the complement-regulated OC differentiation. Samples of 2 × 104 primary WT or C3−/− calvarial OBs were cultured with 2 × 106 WT and C3−/− splenocytes (as source of OC progenitors) together with 1 × 10−8M 1,25(OH)2 vitamin D3. The resultant TRAP-positive cells were counted after 12 days of coculture.
Complement is locally produced and required for efficient OC differentiation in humans. (A) Zymosan-based C3b uptake assays using factor B−/− sera plus 1:5 diluted control media (solid line) or human BM cell–conditioned media (dotted line), showing that BM cell-conditioned media compensated the absence of factor B. (B) Zymosan-based C3b uptake assays using factor D−/− sera plus 1:5 diluted control media (solid line) or human BM cell-conditioned media (dotted line), showing that BM cell-conditioned media compensated the absence of factor D. (C) EshA-hemolytic assays using C5-depleted sera plus 1:5 diluted control media or BM cell-conditioned media, showing that BM cell-conditioned media compensated the absence of C5, therefore inducing C5b-9–mediated hemolysis. (D) Human BM cells were incubated with 1 × 10−8M 1,25(OH)2 vitamin D3 in the presence of placebo (control), C3aRA, C5aRA, or C3aRA·C5aRA, showing that efficient OC differentiation in humans also requires C3aR/C5aR as in mice. Representative results of 2 independent experiments. Data are mean ± SD: *P < .05, #P < .05. (E) Both mesenchymal cells and OC progenitors are involved in the complement-regulated OC differentiation. Samples of 2 × 104 primary WT or C3−/− calvarial OBs were cultured with 2 × 106 WT and C3−/− splenocytes (as source of OC progenitors) together with 1 × 10−8M 1,25(OH)2 vitamin D3. The resultant TRAP-positive cells were counted after 12 days of coculture.
Discussion
In this report, using BM cells from respective complement and complement receptor knockout mice, we demonstrated that complement is required for efficient 1,25(OH)2 vitamin D3–stimulated OC differentiation. More importantly, we found that during OC differentiation, BM cells locally produce all complement components essential for C3a/C5a generation through the alternative pathway of complement activation. In addition, our results indicate that C3aR and C5aR are integrally involved in the process of OC differentiation, and the local production of IL-6 regulated by C5aR/C3aR is at least part of the underlying regulatory mechanism.
When Dr Suda and his colleagues discovered a role for the complement in OC differentiation in 1993,12 no complement-related knockout mouse was available. By adding mAbs against C3, they showed that C3 locally produced by BM cells are required for efficient OC differentiation. However, functional assays using blocking Abs are not as definitive as are assays using a genetic approach. For example, an antibody could directly injure cells after binding to its antigen on the cell surface, especially when complement activation is occurring at the same time, which could complicate data interpretation. By using BM cells from C3−/− mice, we demonstrated that C3 locally produced by BM cells is required for efficient OC differentiation. To further distinguish the relative contribution of osteoblasts and OC progenitors during complement-regulated OC differentiation, we set up cocultures using fetal calvarial osteoblasts and splenocytes from WT and/or C3−/− mice, and assessed their OC generation efficiencies. These coculture experiments showed that while WT spleen cells cocultured with C3−/− OBs showed fewer TRAP+ multinucleated cells, there was an additional reduction in TRAP+ multinucleated cells when C3−/− OC precursors were included in the coculture. These results indicate that complement from both the mesenchymal cells and OC progenitor cells contribute to the regulation of OC differentiation (Figure 7E).
Another important discovery in Dr Suda's report was that C3 locally produced by BM cells, but not systemic C3 produced by hepatocytes, plays a critical role in OC differentiation.12,19 Similarly, we29 and others30 recently showed that local complement produced by both interacting antigen-presenting cells and T cells, but not systemic complement produced in the liver, is important in modulating T-cell responses. These studies suggest that complement locally produced by cells in the microenvironment, even in relatively small quantities, could reach concentrations high enough to play a significant role in regulating cellular function without the involvement of the systemic complement produced in the liver.
As indicated above, for C3 to impact any cell, it first needs to be activated. The results that culture supernatants from WT BM cells under 1,25(OH)2 vitamin D3 stimulation, but not control media, are able to compensate for factor B–, factor D–, or C5-deficient serum in various complement assays clearly indicate that, in addition to C3 production, as reported by others,12,19 these cells produce functional factor B, factor D, and C5 during OC differentiation. These complement components are essential for the alternative pathway of complement activation to activate C3 and C5, and to locally generate ligands for C3 receptors as well as C3a/C5a, ligands for C3aR/C5aR. The results that factor D−/− BM cells generated significantly less TRAP-positive cells than WT BM cells indicate that the alternative pathway is required for local complement activation and efficient OC differentiation from hematopoietic stem cells.
Since C3a and C5a can be generated locally by BM cells during OC differentiation, their receptors, C3aR and C5aR, which are expressed on all cells with the hematopoietic lineage, could also participate in the process, in addition to the C3 receptors (CR1-4) that have been studied before.12 To dissect out the role of C3aR and C5aR during OC differentiation, we subjected BM cells from WT, C3aR−/−, C5aR−/−, and C3aR−/−C5aR−/− mice to OC differentiation assays and, in complimentary experiments, blocked C3aR and/or C5aR activity on WT BM cells during their differentiation with C3aRA and/or C5aRA. The results of these assays suggest that both C3aR and C5aR are integrally involved in OC differentiation, and that C3aR may play a more predominant role than C5aR. One potential criticism of these results could be that the complement effects observed are merely mouse strain-specific effects, which has been indicated in other studies on osteoclastogenesis31,32 . However, the results using human BM cells strongly suggest that these observations are not simply a mouse strain–specific phenomenon (Figure 7).
IL-6 is a hallmark cytokine produced by various types of cells, including monocytes and macrophages, after C3aR/C5aR stimulation. Previous work has shown that IL-6 augments 1,25(OH)2 vitamin D3-induced OC differentiation from BM cells through IL-6R and gp130.33,34 The results showing that C3−/− or C3aR−/−C5aR−/− BM cells produce ∼ 5-fold less IL-6 during differentiation than WT BM cells (Figure 6A) argue that IL-6 production by BM cells during OC differentiation is regulated by C3aR/C5aR. The results showing that supplementation of IL-6 into C3−/− BM cells augmented TRAP-positive cell production (Figure 6B), and that neutralization of IL-6 in WT or C3−/− BM cells stimulated with C3a/C5a efficiently inhibited exogenous complement-induced OC differentiation (Figure 6C-D), support the concept that IL-6 could play an important role in complement-regulated OC differentiation.
Our study, together with the previous work by others,12 indicates that the locally produced complement by BM cells enhances OC differentiation through C3 receptors and C3aR/C5aR, and that IL-6 is mechanistically involved in this process. These results also argue that complement inhibitors that suppress C3a/C5a production or C3aR/C5aR function could, in principle, be developed to suppress OC generation in many clinical situations, such as rheumatoid arthritis or osteoporosis. In fact, inhibition of complement activation has been found to be effective in reducing bone resorption in different models of rheumatoid arthritis,35,36 and mice deficient of C3 or the alternative pathway of complement activation exhibit ameliorated bone damage in similar studies.37,–39 Both of these observations could, at least partially, be explained by findings in this report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Yuanyuan Ma from the University of Alabama at Birmingham, Birmingham, AL, for factor D−/− mice, Dr Michael Holers from the University of Colorado at Denver, Denver, CO, for factor B−/− mice, and Dr Craig Gerard from Harvard University, Cambridge, MA, for C3aR−/− and C5aR−/− mice.
This work was supported by National Institutes of Health grant NS052471 (to F.L.). Z.T. and H.B. were supported, in part, by China National Basic Research Fund grant 2005CB623901 and the Chinese Oversea Postgraduate Program Fellowship (3019-2008624057).
National Institutes of Health
Authorship
Contribution: Z.T. performed all the experiments; F.L. conceptualized and designed the experiments with J.E.D. and H.B.; and F.L. and J.E.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Feng Lin, Institute of Pathology, Case Western Reserve University School of Medicine, 2085 Adelbert Rd, Rm 306, Cleveland, OH 44106; e-mail: feng.lin@case.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal