Abstract
Abstract 672
The favorable results of second generation tyrosine kinase inhibitors (TKIs; nilotinib, dasatinib) in frontline therapy of Philadelphia chromosome (Ph) positive-CML may establish them as new standards in frontline therapy. This depends on the maturing data with the long-term endpoints of PFS and EFS. Different definitions are used to define “progression” and “event” in different studies. This may result in perceived but not real differences in outcomes with various TKIs when comparing trials. In addition, multi-institutional sponsored trial designs may compound the variable definitions: 1) patients taken off study for occurrences other than the defined “progression” or “event” (eg toxicity, intolerance, other) are censored at the time they are off therapy and not coded for progression/event/death once they are off TKI; and 2) such studies have limited capacities to follow up patients for progression/event after they are off drug therapy for more than 30–60 days. Single institutional studies have a potential advantage of continuing to monitor patients for progression/events after they are off the particular protocol TKI.
To analyze the impact of differences in the definitions of PFS and EFS used in the IRIS, ENEST-nd, DASISION, and M.D. Anderson (MDACC) trials on outcome, when these definitions are applied to patients with newly diagnosed CML treated with TKIs on MDACC studies.
435 patients (July 2000-April 2010) with early chronic phase Ph-positive CML treated with imatinib (n=281), nilotinib (n=78), and dasatinib (n=76)were analyzed for outcome using different definitions. Definitions were: 1) PFS- ENEST: progression = accelerated or blastic phase (AP-BP) on nilotinib/imatinib therapy + CML related death on nilotinib/imatinib therapy or within 30 days off therapy; 2) EFS-IRIS: event = progression to AP-BP on imatinib + death of any cause on imatinib + loss of CHR or major CG response; 3) PFS-DASISION: progression = EFS-IRIS definition + WBC increase to more than 20; deaths are coded on dasatinib and within 60 days off dasatinib; 4) EFS MDACC: event = progression to AP-BP + loss of major CG response +resistance/loss of CHR/lack of achievement of response by ELN criteria + off for toxicity + death from any cause on or off therapy (if not counted prior to death as progression/event).
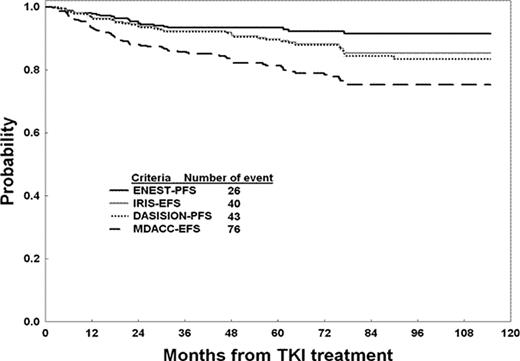
The median follow-up is 67 months (2-116). Of the 435 patients treated, 312 (72%) remain on TKI therapy; 123 (28%) were taken off for the following reasons: resistance/loss of response n = 33; blastic phase on TKI therapy n=6; intolerance/toxicity n= 29; other causes n = 55. Reasons off for the latter 55 patients are: lost to follow – up (n=14); non-compliance (n=11); financial issues (n=8): intercurrent illness (n=7); patient choice (n=5); referral to SCT in chronic phase (n=2); and death from non-CML cause (n=8: 1 complications of surgery, 2 old age, 1 CHF, 1 pneumonia, 2 car accident/suicide, 1 cardiac infarction). So far, 33 patients (7.6%) have died; 8 while on TKI therapy (none from CML; detailed earlier); 2 within 60 days off TKIs (1 AML, 1 renal cancer); and 23 off TKIs for > 60 days. Deaths in the latter 23 were from: 10 post resistance/relapse/BP (accounted for as event/resistance at time off TKI); 10 taken off for toxicity/intolerance (censored at time off; 8 deaths later from CML, 1 post SCT, 1 unknown); 4 off for other illness/non-compliance/lost to FU/pt choice (3 deaths later from CML; 1 from other). Thus, of the 33 deaths, only 19 (8 deaths on TKI + 2 deaths within 60 days + 9 off for resistance/relapse/BP) would be counted as progression/events on the IRIS/ENEST/DASISION studies while 14 would be censored at time off TKI. Based on these 4 definitions, the number of progression/events were: PFS-ENEST 26 progressions; EFS-IRIS 40 events; PFS-DASISION 43 progression/events; EFS-MDACC 76 events. The corresponding 5-year PFS EFS rates were 93%, 90%, 89%, and 81%. (Figure)
With the importance of EFS and PFS in determining whether new TKIs are better than imatinib in frontline therapy, precise and common definitions of these endpoints across randomized clinical trials and single institutional trials are needed. Randomized multi-institutional trials may not collect accurately all events after patients are off TKI therapy for 30–60 days.
Kantarjian:Novartis Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding. O'Brien:Novartis: Research Funding; BMS: Research Funding. Kadia:Novartis: Membership on an entity's Board of Directors or advisory committees. Cortes:Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal