Abstract
Abstract 641
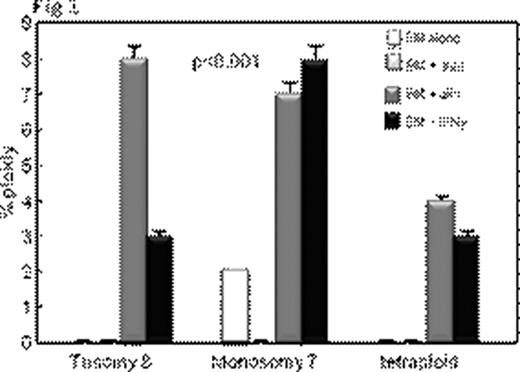
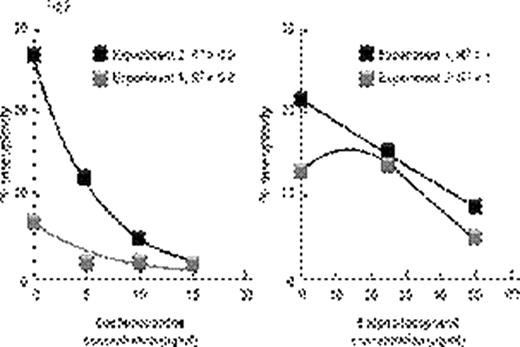
Inflammatory conditions such as ulcerative colitis or Barrett's esophagus predispose to chromosomal instability, microsatellite instability, gene hypermethylation, loss of tumor suppressor genes such as p53 leading to malignant transformation. Aplastic anemia (AA) also shows some features of chronic inflammation – immunosuppressive treatment (IST) responders show persisting activation of Th1 lymphocytes (Sloand et al Blood 2002) and it is well recognized that AA patients can develop clonal hematologic disorders. Bone marrow (BM) at presentation may show clonal abnormalities by fluorescence in situ hybridization (FISH) or SNP array analysis. We postulated that chromosomal abnormalities in severe AA may be a consequence of genomic damage caused by effector T-cells as well as telomere shortening resulting from accelerated cell turnover. Selective survival of aneuploid cells such as trisomy 8 and monosomy 7 in an inflammatory environment may occur subsequently due to upregulation of anti-apoptotic genes. We examined the relationship between inflammation and clonal expansion in bone marrow (BM) by culturing normal bone marrow mononuclear cells (BMMNCs) for two days with mis-matched allogeneic lymphocytes, autologous lymphocytes or interferon gamma (IFN γ) prior to short-term methylcellulose culture. After two weeks, colonies were picked, pooled, and stained with annexin; annexin-negative cells were subjected to FISH and spectral karyotyping (SKY). Telomere length was determined by qPCR. To assess the role of free-radicals in the inflammatory setting, we performed separate studies using the free radical scavengers, alpha-tocopherol, and desferrioxamine. We also cultured BMMNCs in low oxygen (5%) to decrease free radical generation. P53 gene was examined by FISH, sequenced, and protein expression measured by immunoblot. We also examined the in vivo effect of inflammation in the AA mouse model (Bloom ML et al Exp Hematol 2004). Luminex for cytokines was performed on the supernatants of all samples. Annexin negative human BMMNCs showed significant aneuploidy after short-term culture with allogeneic lymphocytes or with IFNγ (Fig 1; N=10; p<0.01). SKY showed aneuploidy in chromosomes 7 and 8. Lymphocytes preincubated with 200ng/mL of cyclosporine prior to co-cultivation with BMMNCs did not induce aneuploidy, nor did autologous lymphocytes or allogeneic lymphocytes separated from bone marrow by a semi-permeable membrane. Aneuploidy-induced by allogeneic lymphocytes was ameliorated by alpha-tocopherol and desferrioxamine in a dose-dependent manner (Fig 2) and eliminated by MHC blocking antibody. BMMNCs cultured in 5% O2 had significantly less aneuploidy after culture with allogeneic lymphocytes than BMMNCs cultured in 21% O2 (mean 4% and 15% aneuploidy respectively N=3;). BMMNCs, the samples cultured with CD8 cells but not CD4 cells developed aneuploidy (N=3; mean 10% and 3% respectively) and had a 36% decrease in telomere length compared to control. P53 protein expression was decreased in BMMNCs cultured with mis-matched lymphocytes, but was upregulated ten-fold by alpha-tocopherol and low O2. Aneuploidy was also detected in samples exposed to H2O2 (N=4) suggesting a role for free radicals. No abnormalities in p53 gene sequence could be detected in any of the cultured BMMNC. Haploinsufficiency for p53 was noted in 70% of the aneuploid cells but none of the diploid cells (N=3). CXCL5 and IL10, IFNγ and TFNα concentrations were greatest in the supernants of BMMNCs cultured with lymphocytes. We assessed whether lymphocyte-infusion induced aneuploidy in the murine model of AA. Mice receiving lymphocyte infusions but not controls showed clonal populations of aneuploid cells by SKY. Chromosomes 11 (containing p53 gene), 19 and 7 clones were most frequently affected. These data suggest that inflammatory changes can facilitate clonal evolution in AA by causing DNA damage, telomere erosion and selection of cells resistant to apoptosis. The relationship of inflammatory changes to clonal progression in AA deserves further study.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2010 by The American Society of Hematology
2010

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal