Abstract
Abstract 5054
The discovery of JAK2 V617F mutation in patients with myeloproliferative disorders (MPD) has opened new perspectives for the development of targeted therapies. We have studied the efficacy of a novel molecule LY2784544 with JAK2 inhibitory activity in the in vitro growth of myeloid progenitors from JAK2 V617F-positive polycythemia vera (PV) patients.
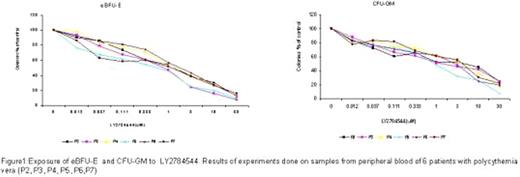
To investigate the efficacy of LY2784544 in the inhibition of endogenous(e)BFU-E and CFU-GM growth in PV patients.
In vitro cultures in semisolid media were performed from peripheral blood mononuclear cells (PBMC) of 6 PV patients who had never received cytoreductive treatment (4 patients with homozygous JAK2 V617F and 2 patients with heterozygous JAK2 V617F). PBMC were suspended in methylcellulose (Methocult. StemCell, Vancouver, Canada) without the addition of EPO and containing 0–30.0 μM LY2784544 drug. Concurrent plates containing EPO were plated as control cultures. The medium was distributed in multidishes and they were incubated at 37° with 5% CO2 and 95% humidity. Hemoglobinized colonies and granulomonocytic colonies were counted on day 14 by standard criteria (BFU-E defined by an aggregate of >50 hemoglobinized cells or three or more erythroid subcolonies and CFU-GM was defined by an aggregate of >50 cells). Each in vitro assay was performed in duplicate. DNA was obtained from peripheral blood granulocytes from each patient to quantify the JAK2 V617F allele burden at the time of culture assay.
LY2784544, at concentrations ranging from 0.03–30.0 μM, inhibited growth of unselected peripheral blood eBFU-E and CFU-GM from PV patients carrying the JAK2 V617F mutation in a dose-dependent manner, although without achieving complete inhibition of all colonies (fig.1).
In vitro studies show that LY2784544 decreases the eBFU-E and CFU-GM growth in therapy-naive JAK2 V617F positive PV patients. Our data suggest that LY2784544 may be a candidate for the treatment of MPD carrying the JAK2 V617F mutation.
Ma:Eli Lilly and Company: Employment. Walgren:Eli Lilly and Company: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal