Abstract
Abstract 5042
Patients with smoldering multiple myeloma (MM) may remain asymptomatic (ASx) for variable amounts of time and are therefore typically monitored without treatment. Chemoprevention trials using thalidomide found prohibitive toxicity and longer follow up is needed for early systemic treatment with lenalidomide/dexamethasone of high risk ASxMM. Based on encouraging preclinical data with bioactive food supplements in MM curcumin (Blood 2003), resveratrol (Blood 2006), and a component of green tea extract (Blood 2006) many patients are already using these agents without definitive proof of efficacy or safety. Preclinically, sphingolipids/glycosides contained in sea cucumbers have also demonstrated antitumor properties including antiangiogenesis direct tumor cytotoxicity, and also of particular relevance to MM, the inhibition of osteoclastogenesis. TBL12, an extract of sea cucumber, has been commercially available since 1981 and used by human subjects as a food supplement without any reported toxicities. We therefore designed a pilot phase II study to determine the safety and efficacy of TBL12 in patients with ASxMM.
Patients were required to have ASxMM with measurable disease, defined as serum m spike ≥ 1 g/dL and/or urine m spike ≥ 200 mg/24 hours. If non-secretory, patients were required to have abnormal free light chains (FLC). A total of 20 patients with ASxMM were given TBL12, formulated as a liquid gel (manufactured by Unicorn Pacific Corporation, IND 103,543) to be kept frozen until the time of consumption. Patients ingested 2 units of 20 ml twice per day, for a total of 80 units per day. Disease parameters were monitored monthly and treatment was continued until progression of disease (PD).
23 patients were screened, with 3 failures, and the remaining 20 patients proceeded with study treatment. The median age of the patient was 58 years (range 22–75), with 11 males and 9 females. The phenotypes were 14 IgG, 5 Ig A, and one kappa light chain. Generally, this was a high risk ASxMM population, with 14 patients having a serum m spike ≥ 3 g/dl and bone marrow plasma cells (PC) ≥ 10%. The median bone marrow PC involvement for all patients was 38% (range 10 to 90). (With the additional high risk criteria of a FLC ratio <0.125 or >8, 13 patients were high risk.) Of the remaining 6 patients, all had immunoparesis and 4 had markedly elevated FLC ratios (range 307-incalculable) and the remaining 2 patients had 9.2 g of bence jones proteinuria (BJP) and an IgA phenotype.
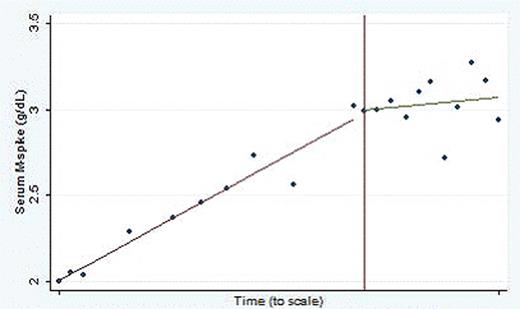
Compliance was excellent and the treatment was well tolerated with only grade 1 nausea. There was one SAE, a pneumococcal pneumonia requiring admission, which was felt to be unrelated to study treatment. A total of 11 patients remain on treatment, having completed a median of 11 monthly cycles of TBL12 (range 6–17 cycles). The best response to date has been stable disease. Notably, one high risk patient demonstrated a flattening in the rate of rise of the m spike concordant with the initiation of study treatment (see Figure 1).
6 patients came off study for PD after a median of 6.5 cycles (range 2–10). The reasons for PD include: 1 hypercalcemia, 1 acute renal insufficiency (after 2 cycles with 9.2g BJP at screening), 1 for anemia (after 3 cycles with 90% marrow PC at screening), and 1 for a new bony lesion on MRI. 2 patients withdrew consent after cycle 6 and 8, and 1 was removed after cycle 13 due to investigator discretion after the pneumonia SAE.
-1 year I + 1 year
TBL 12 initiated
In this pilot study of high risk ASxMM patients, TBL12 was well tolerated and 11 patients (65%)remain on treatment. The expected rate of progression for this population from diagnosis is 52% at 2 years, however, the median time to progression has not been yet reached in this study. Additional follow up is required and data will be updated at the annual meeting. The decrease in the rate of rise in the m-spike in a high risk patient concomitant with the initiation of study treatment is suggestive of a biologic effect of TBL12 in MM and warrants further study of TBL 12 in a larger cohort of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal