Abstract
Abstract 4837
Many hematological abnormalities have been reported during the neonatal period in infants with Down syndrome (DS). Neonates with DS are predisposed to developing transient abnormal myelopoiesis (TAM) and acute megakaryblastic leukemia (AMKL). However, there is a paucity of data on hematological aberrations and GATA1 gene mutations in neonates with DS in East Asian populations. We therefore investigated the spectrum of hematological abnormalities in infants and children with DS in one such population, and analyzed the frequency and pathophysiological role of GATA1 mutations in DS-TAM and DS-AMKL in this group.
A total 148 patients with DS were identified from January 2000 to December 2009. Of these, 109 had had blood drawn for a complete blood count (CBC) with differential. There were 37 neonates (°Â4 weeks of age), 55 children (<18 years of age), and 17 adults °Ã 18 years of age) in the study. A comprehensive molecular study of the GATA1 gene was performed using PCR, RT-PCR and direct sequencing for all six exons (7,757 bp) and cDNA sequence of GATA1 gene.
Thrombocytopenia was most common hematological abnormality (35%) in the neonates. Leukocytosis and polycythemia were each detected in 4 cases (11%). Blasts were identified in the differential count of 6 (16%) neonates. Of the 55 children with DS, 11 (20%) had thrombocytopenia, 10 (18%) had anemia, and 7 (13%) had neutropenia (< 1,500/uL). There were also 4 (7%) cases of AMKL and 2 (4%) cases of acute lymphoblastic leukemia in the children with DS. GATA1 mutations were identified in all of the TAM samples and in 2 of the 3 AMKL samples, but were not detected in the 1 remission sample or the 2 samples from normal DS children.
East Asian DS neonates showed distinctive hematological abnormalities such as uncommon neutrophilia, relatively common neutropenia, and increased prevalence of TAM compared with reports from Western countries. The GATA1 mutation analysis may offer the potential to accelerate and refine the diagnosis of TAM and AMKL, and to accurately measure the kinetics of the mutant GATA1 clone as a tool for minimal residual disease measurement because virtually all cases of TAM and AMKL harbored this mutation.
Hematological problems recognized among 37 neonates and 55 children with Down syndrome
| Hematological problem . | Neonates (n=37) . | Children (n=55) . | |||
|---|---|---|---|---|---|
| Proportion with this problem recognized . | TAM (n=5, 13.5%) . | Proportion with this problem recognized . | Significant hematological abnormalities . | ||
| AMKL (n=4) . | ALL (n=2) . | ||||
| Thrombocytopenia | 13 (35.1%) | 3 | 11 (20.0%) | 4 | 2 |
| Mild (10-15 ×10^3/uL) | 7 (18.9%) | 2 | 4 (7.3%) | ||
| Moderate (5-10 ×10^3/uL) | 5 (13.5%) | 4 (7.3%) | 2 | 1 | |
| Severe (<5 ×10^3/uL) | 1 (2.7%) | 1 | 3 (5.5%) | 2 | 1 |
| a Anemia | 10 (18.2%) | 1 | 2 | ||
| b Leukocytosis | 4 (10.8%) | 4 | 3 (5.5%) | 1 | |
| c Neutrophilia | 1 (2.7%) | ||||
| d Neutropenia | 6 (16.2%) | 7 (12.7%) | |||
| e Polycythemia | 4 (10.8%) | ||||
| Thrombocytosis | 1 (2.7%) | 1 | |||
| Hematological problem . | Neonates (n=37) . | Children (n=55) . | |||
|---|---|---|---|---|---|
| Proportion with this problem recognized . | TAM (n=5, 13.5%) . | Proportion with this problem recognized . | Significant hematological abnormalities . | ||
| AMKL (n=4) . | ALL (n=2) . | ||||
| Thrombocytopenia | 13 (35.1%) | 3 | 11 (20.0%) | 4 | 2 |
| Mild (10-15 ×10^3/uL) | 7 (18.9%) | 2 | 4 (7.3%) | ||
| Moderate (5-10 ×10^3/uL) | 5 (13.5%) | 4 (7.3%) | 2 | 1 | |
| Severe (<5 ×10^3/uL) | 1 (2.7%) | 1 | 3 (5.5%) | 2 | 1 |
| a Anemia | 10 (18.2%) | 1 | 2 | ||
| b Leukocytosis | 4 (10.8%) | 4 | 3 (5.5%) | 1 | |
| c Neutrophilia | 1 (2.7%) | ||||
| d Neutropenia | 6 (16.2%) | 7 (12.7%) | |||
| e Polycythemia | 4 (10.8%) | ||||
| Thrombocytosis | 1 (2.7%) | 1 | |||
Abbreviations: AMKL = acute megakaryoblastic leukemia; ALL = acute lymphoblastic leukemia
Hemoglobins < lower limit of reference range for postnatal age [Nelson Textbook of Pediatrics, 18th ed.]
WBC counts > upper limit of normal for postnatal age [Nelson Textbook of Pediatrics, 18th ed.]
Blood neutrophil counts > upper limit of reference range for postnatal age [J Perinatol 2008;28: 275–281]
Blood neutrophil counts < lower limit of reference range for postnatal age [J Perinatol 2008;28: 275–281]
Hemoglobins or hematocrits > upper limit of reference range for postnatal age [pediatrics 2009;123: e333-7]
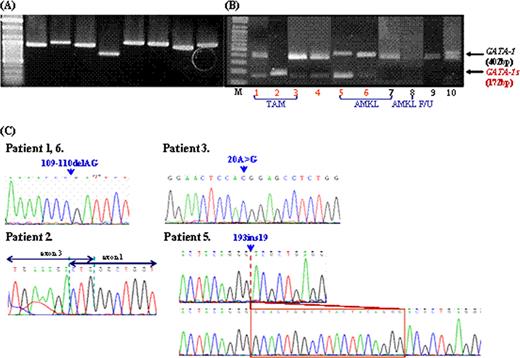
Molecular studies of the GATA1 gene. The GATA1 gene was amplified using gDNA and cDNA genomic DNA and cDNA. (A) PCR amplified products of GATA1 gene using 8 overlapping primer sets. (B) RT-PCR amplified products of exon 2 of GATA1, GATA1 mRNA (400 bp), and GATA1s mRNA (172bp) are shown. (C) Reverse partial chromatogram of GATA1 gene in exon 2. Direct sequencing data showed GATA1 mutations from 109–110 del AG in exon 2 in patient 1 with TAM and patient 6 with AMKL, 1–239del239bp in patient 2 with TAM, 20A>G in patient 3 with TAM, and 193ins19bp in patient 5 with AMKL.
Molecular studies of the GATA1 gene. The GATA1 gene was amplified using gDNA and cDNA genomic DNA and cDNA. (A) PCR amplified products of GATA1 gene using 8 overlapping primer sets. (B) RT-PCR amplified products of exon 2 of GATA1, GATA1 mRNA (400 bp), and GATA1s mRNA (172bp) are shown. (C) Reverse partial chromatogram of GATA1 gene in exon 2. Direct sequencing data showed GATA1 mutations from 109–110 del AG in exon 2 in patient 1 with TAM and patient 6 with AMKL, 1–239del239bp in patient 2 with TAM, 20A>G in patient 3 with TAM, and 193ins19bp in patient 5 with AMKL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal