Abstract
Abstract 4780
Histone deacetylation maintains chromatin in a condensed configuration preventing gene expression in eukaryotic cells. The deacetylation reaction is catalyzed by enzymes of the histone deacetylase (HDAC) superfamily, which perform their functions as multiprotein complexes including at least 2 HDAC isoforms, DNA docking factors (transcription factors and methyl-binding proteins) and protein kinases (PKC and Erk). The well established role of HDACs in gene silencing has suggested studies to identify HDAC inhibitors (HDACi) that, by re-activating γ-globin expression, might treat the anemia due to insufficient β-globin expression (Cao et al Blood 103:701, 2004). Over the years several HDACi have been documented to induce γ-globin expression in human erythroid cultures, adult baboons, and β-thalassemia and sickle cell patients. Among those, Class I HDACi, and in particular those that inhibit HDAC3, appear to be more potent as γ-globin gene activators (Mankidy et al, Blood 108:3179, 2006).
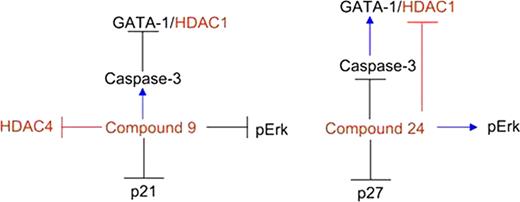
We have recently identified two new HDACi (compound 9 and 24) which both improved maturation and reactivated γ-globin expression in β°-thalassemic erythroblasts in vitro (Mai et al Mol Pharmacol 72:111, 2007). Compound 24 inhibits both class I (HDAC1 ID50 =0.2 μ M) and class IIa (HDAC4 ID50=0.6 μ M) HDAC. Compound 9 is a class IIa specific inhibitor (HDAC4 ID50=20 μ M) and does not affect HDAC1 activity but is a more potent γ-globin inducer than compound 24. This observation suggests that HDACi may also affect HDAC activity through indirect effects which alter overall complex activity. To clarify possible off-target effects of Class II and Class I/IIa inhibitors and their consequences for erythroid maturation, we analysed expression and activity of different HDAC isoforms during maturation of normal human erythroblasts in vitro at baseline and with treatment with compounds 9 and 24. The proteins studied included GATA1 (the major transcription regulator of erythroid maturation), p21/p27kip1, two cyclin D dependent kinase inhibitors which favor maturation, Caspase 3 (the protease which specifically cleaves GATA1) and Erk (a component of the HDAC complex).
During normal erythroid maturation (without HDACi), all the HDAC isoforms were expressed at the mRNA and protein levels. Immunoprecipitation studies followed by determination of HDAC activity indicated that the activities which changed most during maturation are those of HDAC1 (class I), increased by 2-fold, and HDAC5 (class IIa), decreased by 2-fold. In addition, co-immunoprecipitation studies revealed an increase in the association between HDAC1 and GATA1 with erythroid maturation. Changes in the expression of key regulatory proteins were observed with normal erythroid maturation: activation of Caspase 3 decreased with resultant increase in GATA-1, and phosphorylation of pErk decreased while expression of p21 and p27 increased.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal