Abstract
Abstract 4122
We have previously identified sole +9, 13q- or 20q- as “favorable” and sole +8 or complex karyotype as “unfavorable” cytogenetic abnormalities in primary myelofibrosis (PMF) (Blood 2010; 115: 496). The purpose of the current study, which includes more than twice the number of patients included in previous studies, was to identify additional prognostically-relevant cytogenetic abnormalities in PMF and refine cytogenetic risk categorization for overall and leukemia-free survival.
Clinical and laboratory data were collected from consecutive patients with PMF seen at our institution and in whom cytogenetic information at or within 1 year of diagnosis was available. Diagnosis of PMF and acute myeloid leukemia were according to the World Health organization (WHO) criteria.
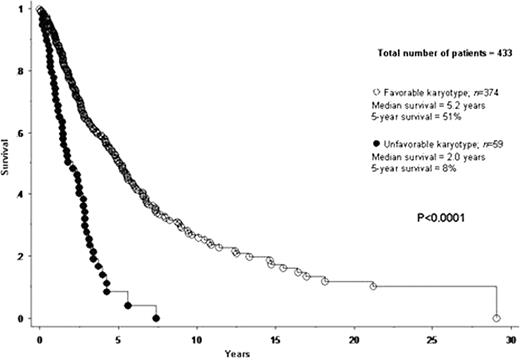
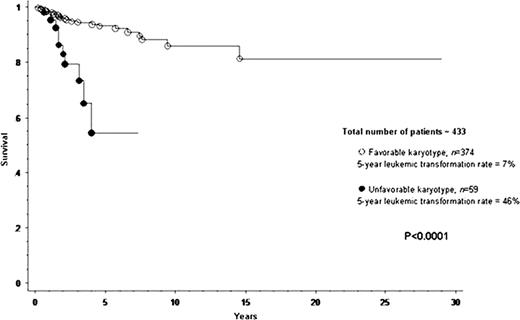
A total of 433 patients with PMF were included in the current study. Median age at diagnosis was 65 years. IPSS risk distributions were low in 12% of patients, intermediate-1 in 25%, intermediate-2 in 24% and high in 39%. JAK2V617F mutational frequency was 60%. Cytogenetic findings were normal in 275 (64%) patients. Among the 158 (36%) patients with abnormal karyotype, 109 (69% of abnormal cases) represented sole abnormalities, 23 (15%) two abnormalities and 26 (17%) three or more (i.e. complex) abnormalities. In an effort to identify cytogenetic categories of similar prognosis, each one of 12 operational cytogenetic categories was separately compared with both normal and complex karyotype. Accordingly, we were able to devise a two-tired cytogenetic risk stratification with highly significant differences in overall and leukemia-free survival (Figures 1 and 2): unfavorable (complex karyotype or sole or two abnormalities that include +8, -7/7q-, i(17q), inv(3), -5/5q-, 12p- or 11q23 rearrangement) and favorable (all other cytogenetic findings including normal karyotype). Median survivals of patients with favorable and unfavorable karyotype were 5.2 and 2.0 years, respectively (p<0.0001). The corresponding 5-year survival rates were 51% and 8% (HR 3.1, 95% CI 2.2–4.3; p<0.0001). Multivariable analysis confirmed the IPSS-independent prognostic value of cytogenetic risk categorization (p<0.0001; HR 2.1, 95% CI 1.5–3.1) and also identified thrombocytopenia (platelet count < 100 × 109/L) as another independent predictor of inferior survival (p<0.0001; HR 1.9; 95% CI 1.4–2.6). A similar multivariable analysis showed that cytogenetic risk profile (p=0.001; HR 4.1, 95% CI 1.7–9.6) and platelet count (p=0.04; HR 2.3, 95% CI 1.0–5.0), but not IPSS (p=0.27), predicted leukemia-free survival; the 5-year leukemic transformation rates for unfavorable and favorable karyotype were 46% and 7%, respectively (HR 5.5, 95% CI 2.5–12.0; p<0.0001). These results, in terms of both overall and leukemia-free survival analysis, did not change when patients receiving allo-SCT were censored at the time of their transplant. Among patients with favorable karyotype; the incidences of leukopenia and thrombocytopenia were highest in patients with sole 20q- (41% and 38%, respectively) and lowest in those with sole 13q- (0% and 0%, respectively).
The current study provides the rationale and necessary details for incorporating cytogenetic findings and platelet count in future prognostic models for PMF. The study also revealed a highly significant association between sole 20q- and both leukopenia and thrombocytopenia, which suggests a 20q- haploinsufficient gene effect.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal