Abstract
Abstract 41
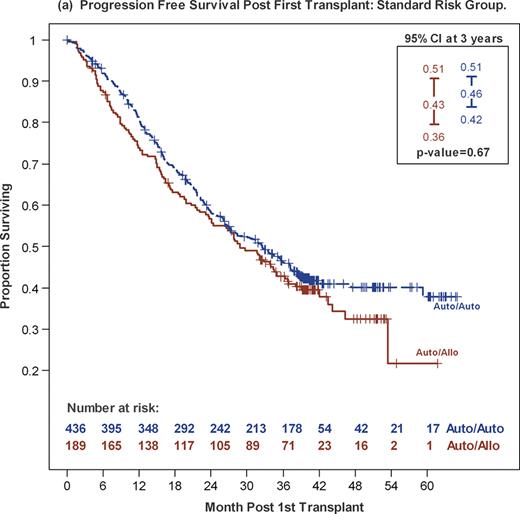
AuHCT improves survival in patients with MM, but disease relapse and progression remain a challenge. Both tandem AuHCT and post transplant maintenance therapy improve progression-free survival (PFS). Alternatively, allogeneic HCT has the potential to reduce disease progression through a graft-versus-myeloma effect. Use of nonmyeloablative conditioning regimens allows the latter approach to be used with reduced treatment-related mortality (TRM). BMT CTN 0102 was a multicenter phase III trial that biologically assigned patients with MM to auto-auto using melphalan 200mg/m2 (MEL 200) conditioning or an auto-allo approach using MEL 200 followed by alloHCT with 2 Gy total body irradiation. Graft-versus-disease (GVHD) prophylaxis was cyclosporine and mycophenolate mofetil. The primary endpoint was 3-year progression free survival (PFS). Between December 2003 and March 2007, 710 patients from 43 US centers were enrolled. Patients were assigned to the auto-allo arm based on availability of an HLA-matched sibling donor at time of enrollment. Patients in the auto-auto arm were further randomized to thalidomide and dexamethasone (Thal-Dex) for 1 year or observation (obs). Among 625 patients with SR MM (absence of chromosome 13 deletion by metaphase karyotyping and β-2 microglobulin ≤ 4mg/L), 436 were assigned to auto-auto (217 Thal-Dex, 219 obs) and 189 to auto-allo. Compliance with Thal-Dex was poor, with 84% of patients not completing prescribed therapy. PFS and overall survival (OS) between the Thal-Dex and obs cohorts were equal and these arms were pooled for the primary analysis. The auto-auto and auto-allo groups differed in age (median 55y vs. 52y, p =0.01) and time between first and second transplants (median 98d vs 105d, p =0.02), but were otherwise balanced. Complete and near complete (CR+nCR) response rates at study entry were 24% for both groups. Three-year PFS was 46% and 43% (p=0.67) and 3-year OS was 80% and 77 % (p=0.19) for the auto-auto and auto-allo groups, respectively. Corresponding probabilities for 3-year progression/relapse were 50% and 46% (p=0.8) and for 3-year TRM were 4% and 11% (p=0.04). Among auto-allo patients, probabilities of grade III-IV acute and chronic GVHD were 9% and 47%, respectively. Eighty-two percent of patients in each arm received the assigned second transplant. Among 522 patients who received their second transplant, 3-year PFS was 47% and 44% (p=0.89) with auto-auto and auto-allo, respectively. Disease response rates at day 56 after second HCT were: 50% very good partial response (VGPR) or better and 40% CR+nCR in the auto-auto group; and 49% (VGPR or better, p=0.8) and 48% (CR+nCR,p=0.12) in the auto-allo group. In conclusion, there were no differences in 3-year PFS and OS between patients receiving auto-auto or auto-allo. Potential benefits of graft-versus-myeloma to reduce disease progression or relapse were offset by increased TRM. Thal-Dex maintenance did not improve PFS or OS, likely due to poor tolerability of this regimen. At 3 years, the auto-allo approach for SR MM had no added benefit compared to tandem AuHCT.
| . | auto-auto (95% CI) . | auto-allo (95% CI) . | p-value . |
|---|---|---|---|
| Day 56 Overall Response1 | 65% | 66% | 0.9 |
| CR | 26% | 35% | |
| nCR | 14% | 13% | |
| VGPR | 10% | 1% | 0.07 |
| PR | 14% | 17% | |
| 3-year Relapse/Progression | 50% (46–55%) | 46% (39–53%) | 0.9 |
| 3-year TRM | 4% (2–6%) | 11% (8–18%) | 0.04 |
| 3-year PFS | 46% (42–51%) | 43% (36–51%) | 0.67 |
| 3-year OS | 80% (77–84%) | 77% (72–84%) | 0.19 |
| . | auto-auto (95% CI) . | auto-allo (95% CI) . | p-value . |
|---|---|---|---|
| Day 56 Overall Response1 | 65% | 66% | 0.9 |
| CR | 26% | 35% | |
| nCR | 14% | 13% | |
| VGPR | 10% | 1% | 0.07 |
| PR | 14% | 17% | |
| 3-year Relapse/Progression | 50% (46–55%) | 46% (39–53%) | 0.9 |
| 3-year TRM | 4% (2–6%) | 11% (8–18%) | 0.04 |
| 3-year PFS | 46% (42–51%) | 43% (36–51%) | 0.67 |
| 3-year OS | 80% (77–84%) | 77% (72–84%) | 0.19 |
Overall Response: complete response (CR) + near CR(nCR) + very good partial response (VGPR) + PR
Krishnan:Celgene: Speakers Bureau. Stadtmauer:Celgene: Speakers Bureau. Comenzo:Millenium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Elan Pharmaceuticals: Consultancy; Genzyme: Research Funding; Celgene: Research Funding; Ortho: Research Funding. Hari:Celgene: Research Funding. Qazilbash:Celgene: Speakers Bureau. Vesole:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Giralt:Celgene: Honoraria, Speakers Bureau; Millenium: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal