Abstract
Abstract 3856
Cell therapy with MSCs appears to be a promising method for treatment of different disorders. Bone marrow and fat tissue are both considered as a prospective source of MSCs for therapeutic applications. However, the differences in functional properties of specific populations of MSC derived from these two tissues of the same patient are still poorly investigated.
Patients and methods: 23 cardiovasular patients and 4 healthy donors were involved in this study. All patients were enrolled in programme funded by EU FP7. MSC cultures from BM (BM-MSC) and subcutaneous adipose (F-MSC) of the same patient were evaluated in successive passages for immunophenotype (FACS analysis), frequency of colony-forming units (CFU) in MSC population, frequency of adipo- and osteo-progenitors (CFU-Ad, CFU-Ost) in the same population and for dynamic of changes in these properties with successive passage. CFU, CFU-Ad and CFU-Ost were studied by limiting dilution assay followed by induction of adipo- or osteo- differentiation as described (Mitchell et al. Stem Cells 2006) with some modifications. Cell suspension was serially diluted two folds across the 8 columns of 96-well plates, resulting in columns containing from 50 to 0,39 cells per well. After 10 days of culture the number of positive and negative wells was determined for each cell concentration and CFU frequency was calculated. Then plates were induced to undergo adipogenesis and osteogenesis and CFU-Ad was determined by Oil Red staining and CFU-Ost by Alizarin Red staining after 14 and 21 days respectively. All calculations were performed as for CFU.
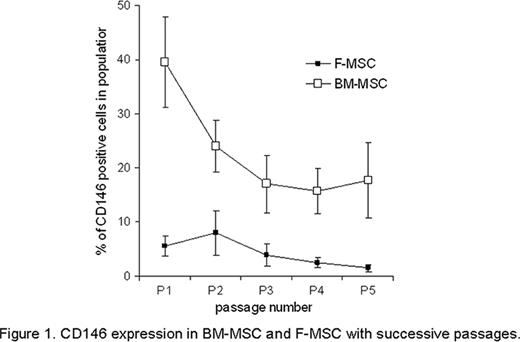
Significant difference was observed in immunophenotype of MSC derived from different tissues of the same donor: while both BM- and F-MSC were positive for stromal cell-associated markers CD105, CD90 and CD73 and were negative for hematopoietic lineage cells markers CD34, CD19, CD14, CD45, the population of CD146+ cells was more abundant in BM-MSC than in F-MSC at passage 1 (39,6%± 8,3% vs 5,6 %± 1,8%; p<0,005), this population of cells further declined in successive passages by passage 5 (17,7%± 6,9% in BM-MSC vs 1,5%± 0,65% in F-MSC; p<0,01); (Figure 1). To further characterize MSC derived from BM and F we studied the frequency of CFU at passages P1-P4, and CFU-Ad and CFU-Ost at passages 2 and 4. Results are shown in Tables 1 and 2.
CD 146 expression in Bm-MSC and F-MSC with successive passages.
CD 146 expression in Bm-MSC and F-MSC with successive passages.
Frequency of CFU at successive passages in BM-MSC and F-MSC
| . | Assay . | P1 . | P2 . | P3 . | P4 . |
|---|---|---|---|---|---|
| BM-MSC | CFU | 30,6%±4,9(n=10) | 23,5%±4,0 (n=8) | 15,3%± 3,7 (n=6) | 8,0%±1,5 (n=9) |
| F-MSC | CFU | 13,54%±1,7 (n=8) | 19,1%±2,8 (n=14) | 21,9%±3,3 (n=14) | 28,6%±3,0 (n=14) |
| . | Assay . | P1 . | P2 . | P3 . | P4 . |
|---|---|---|---|---|---|
| BM-MSC | CFU | 30,6%±4,9(n=10) | 23,5%±4,0 (n=8) | 15,3%± 3,7 (n=6) | 8,0%±1,5 (n=9) |
| F-MSC | CFU | 13,54%±1,7 (n=8) | 19,1%±2,8 (n=14) | 21,9%±3,3 (n=14) | 28,6%±3,0 (n=14) |
Frequency of CFU-Ad and CFU-Ost at passages 2 and 4 in F-MSC and BM-MSC
| F-MSC . | P2 . | P4 . |
|---|---|---|
| CFU-Ad assay | 13,87% ± 3,4 (n=7) | 12,78 ± 3,45 (n=7)** |
| CFU-Ost assay | 16,92% ± 3,56 (n=8) | 15,45% ± 4,73 (n=8)* |
| BM-MSC | P2 | P4 |
| CFU-Ad assay | 10,2% ± 1,6 (n=11) | 3,27 ± 1,11 (n=11)** |
| CFU-Ost assay | 18,6% ± 4,74 (n=7) | 4,57% ± 1,62 (n=8)* |
| F-MSC . | P2 . | P4 . |
|---|---|---|
| CFU-Ad assay | 13,87% ± 3,4 (n=7) | 12,78 ± 3,45 (n=7)** |
| CFU-Ost assay | 16,92% ± 3,56 (n=8) | 15,45% ± 4,73 (n=8)* |
| BM-MSC | P2 | P4 |
| CFU-Ad assay | 10,2% ± 1,6 (n=11) | 3,27 ± 1,11 (n=11)** |
| CFU-Ost assay | 18,6% ± 4,74 (n=7) | 4,57% ± 1,62 (n=8)* |
p<0,002
p<0.05
These data support the idea that in vitro expanded BM- and F- derived MSC differ in their properties. While the frequency of CFU in BM derived MSC population declined by as much as 4-fold by passage 4, there was 2-fold increase in frequency of CFU in Ad-MSC over the same period of culturing and this frequency remain unchanged up to passage 7 (data not shown). These data are consistent with our observation that cultures of BM-MSC showed first signs of senescence (senescence-associated β-galactosidase activity) as early as at passage 4,3±0.5 while the first signs of senescence in Ad-MSC cultures were seen at passage 6,67±0,66 (p<0,02). We assume that early decline in CFU frequency in BM-MSC in vitro is because of cell senescence. Importantly, the difference in CFU-Ad and CFU-Ost was discovered: frequency of both declined significantly in BM-MSC by passage 4, while frequency of Ad- and Ost- lineage progenitors remain unchanged in F-MSC cultures.
For a first time a broad study was performed to compare MSC derived from BM and adipose tissues for immunophenotype, self-renewal and differentiation potential. We have found that BM-MSC and F-MSC differ significantly in all these characteristics. Differences in BM- and F-MSC could be explained by their ontogeny and/or different microenvironment in “parent” tissue. These variations could affect their efficacy in different therapeutic applications.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal