Abstract
Abstract 3701
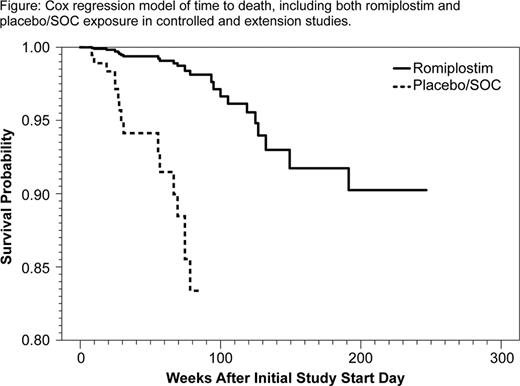
ITP is an autoimmune disease characterized by increased platelet destruction and suboptimal platelet production. ITP pts are at increased risk of bleeding, and those with persistently low platelet counts are at increased risk of fatal bleeding (Cohen, Arch Intern Med, 2000). Romiplostim increases platelet production and offers an alternative treatment option to chronic immune suppression in pts with ITP. We analyzed mortality rates in ITP pts who participated in randomized, controlled romiplostim clinical studies (in which pts received either romiplostim or placebo/standard of care [SOC]) and their subsequent time in an open-label extension study (in which all pts received romiplostim). Methods: Data were pooled from two 24-week placebo-controlled phase 3 studies and a 52-week SOC-controlled study, which included an off-treatment safety follow-up period for those pts who did not enter the extension study. Additional analyses included data from pts' subsequent time in an extension study. The Cox regression model was used to estimate mortality rates. Results: In total, 354 pts (238 romiplostim, 41 placebo, 75 SOC) from the controlled studies were included in the analysis. Of these, 238 enrolled into the extension study (187 romiplostim, 33 placebo, 18 SOC). At the time of enrolment into the controlled study, the median age was 55 years (Q1 –Q3, 42 – 68), and 22% were splenectomized; their median time since diagnosis was 3.5 years (range, 0.02 – 45); pts had received a median of 3 prior ITP therapies (range, 1 – 9), and 24% were receiving baseline concurrent ITP treatment. Pts were followed for up to 87 weeks in the controlled studies; 0.8% (2/238) of pts in the romiplostim arm died and 6.9% (8/116) of pts in the placebo/SOC arm died. Pts who entered the extension study were followed for up to 215 weeks; 5.9% (14/238) of pts died during this time. Pts died from various causes (see Table). The median age of the pts who died was 73 years (Q1 – Q3, 58 – 78). When data from the controlled studies were pooled, mortality rates in the romiplostim arm were approximately 5-fold less than those in the placebo/SOC arm (HR, 0.187; 95% CI, 0.048–0.931; p=0.04). The survival benefit in the romiplostim arm was maintained when time spent in the extension study was added to the analysis (HR, 0.120; 95% CI, 0.035–0.410; p=0.0007) [see Figure]. Factors predicting increased mortality risk in a multiple Cox regression model were: treatment with placebo/SOC vs romiplostim (p<0.0001), age ≥ 65 years (p=0.002), greater number of prior ITP treatments (p=0.064), and concurrent baseline ITP treatment (p=0.016). Furthermore, there was a positive correlation between the grade of worst bleeding event and risk of death (p<0.0001), and there was an association between mortality and experiencing a bleeding event with a worst grade of ≥2 (p=0.0154), ≥3 (p=0.0002), and ≥4 (p=0.0002). Conclusions: In this large retrospective analysis of an ITP pt study population, the mortality rate was significantly lower in pts receiving romiplostim than in those receiving placebo or other ITP therapies. Associations were found between mortality risk and age, prior treatment with other ITP therapies, and on-study bleeding; however, there was no clear pattern of disease-related or treatment-related cause of death, and determining the mechanism for increased survival in the romiplostim arm requires further study.
List of cause of death
| Study Treatmenta . | Age (years) . | Time since ITP diagnosis (years) . | Cause of death . | |
|---|---|---|---|---|
| Controlled studies | Placebo | 70 | 6 | Pneumonia primary atypical |
| Placebo | 51 | 8 | Pulmonary embolism | |
| Placebo | 52 | 21 | Cerebral hemorrhage | |
| Romiplostim | 80 | 1 | Hemorrhage intracranial | |
| SOC | 49 | 0.1 | Hepatic neoplasm malignant | |
| SOC | 71 | 0.01 | Cardio-respiratory arrest | |
| SOC | 73 | 5 | Hepatic failure | |
| SOC | 77 | 7 | Lung cancer metastatic | |
| Romiplostim | 73 | 2 | Pneumonia | |
| SOC | 83 | 10 | Left ventricular failure | |
| Extension study | 48 | 9 | Hepatic neoplasm malignant | |
| 88 | 12 | Cardiac failure congestive | ||
| 82 | 3 | Meningitis listeria | ||
| 35 | 1 | Pneumonia streptococcal | ||
| 47 | 1 | Pneumococcal sepsis | ||
| 69 | 0.2 | Cardiac arrest | ||
| 73 | 14 | Myocardial infarction | ||
| 79 | 0.04 | Myocardial infarction | ||
| 78 | 1 | Myocardial infarction | ||
| 62 | 6 | Angina unstable | ||
| 60 | 6 | Narcotic overdose | ||
| 76 | 15 | Cardiac tamponade | ||
| 78 | 0.4 | Cardiac failure congestive | ||
| 76 | 5 | Lung neoplasm malignant | ||
| Study Treatmenta . | Age (years) . | Time since ITP diagnosis (years) . | Cause of death . | |
|---|---|---|---|---|
| Controlled studies | Placebo | 70 | 6 | Pneumonia primary atypical |
| Placebo | 51 | 8 | Pulmonary embolism | |
| Placebo | 52 | 21 | Cerebral hemorrhage | |
| Romiplostim | 80 | 1 | Hemorrhage intracranial | |
| SOC | 49 | 0.1 | Hepatic neoplasm malignant | |
| SOC | 71 | 0.01 | Cardio-respiratory arrest | |
| SOC | 73 | 5 | Hepatic failure | |
| SOC | 77 | 7 | Lung cancer metastatic | |
| Romiplostim | 73 | 2 | Pneumonia | |
| SOC | 83 | 10 | Left ventricular failure | |
| Extension study | 48 | 9 | Hepatic neoplasm malignant | |
| 88 | 12 | Cardiac failure congestive | ||
| 82 | 3 | Meningitis listeria | ||
| 35 | 1 | Pneumonia streptococcal | ||
| 47 | 1 | Pneumococcal sepsis | ||
| 69 | 0.2 | Cardiac arrest | ||
| 73 | 14 | Myocardial infarction | ||
| 79 | 0.04 | Myocardial infarction | ||
| 78 | 1 | Myocardial infarction | ||
| 62 | 6 | Angina unstable | ||
| 60 | 6 | Narcotic overdose | ||
| 76 | 15 | Cardiac tamponade | ||
| 78 | 0.4 | Cardiac failure congestive | ||
| 76 | 5 | Lung neoplasm malignant | ||
Treatment received at time of death; all pts in the extension study were receiving romiplostim.
Cox regression model of time to death, including both romplostim and placebo/SOC exposure in controlled and extension studies.
Cox regression model of time to death, including both romplostim and placebo/SOC exposure in controlled and extension studies.
Gernsheimer:Amgen Inc.: Consultancy; Cangene: Consultancy; Baxter: Honoraria; Shionogi Corp.: Research Funding. Kuter:Amgen Inc.: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; ONO: Consultancy, Research Funding; Eisai: Consultancy, Research Funding. Cines:Amgen Inc.: Consultancy; GlaxoSmithKline: Consultancy. Stasi:Amgen Inc.: Honoraria, Speakers Bureau; GlaxoSmithKline: Honoraria, Speakers Bureau. Godeau:Amgen France: Consultancy, Research Funding; Roche France: Consultancy, Research Funding; LFB France: Consultancy. Guo:Amgen Inc.: Employment, Equity Ownership. Franklin:Amgen Inc: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal