Abstract
Abstract 3695
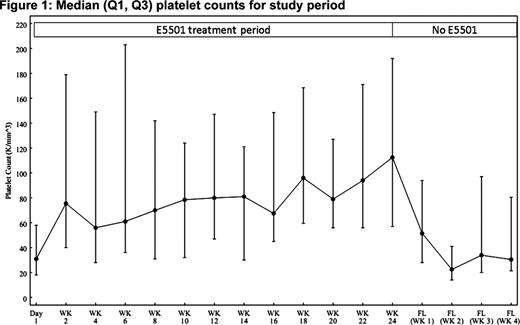
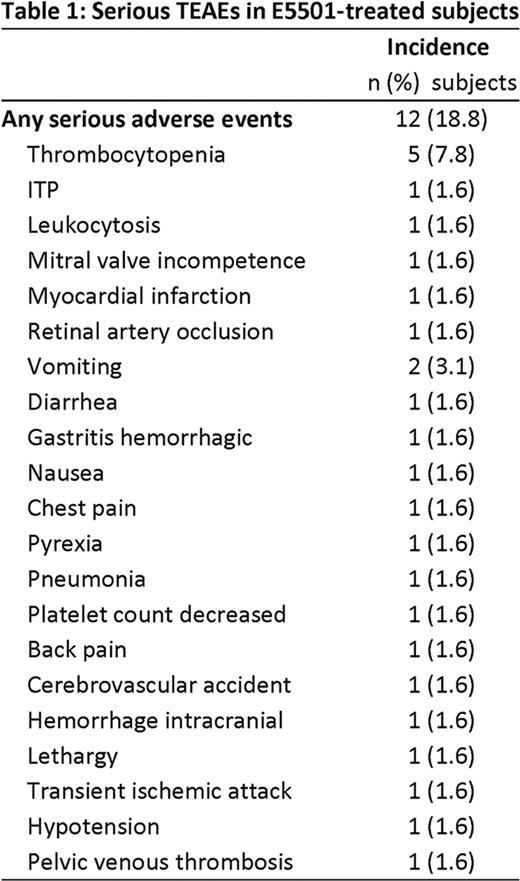
The thrombopoietin (TPO) receptor agonist E5501 (AKR501) is one of a new class of oral TPO agents which increase platelet production. In a recent multicenter, randomized, double-blind, placebo-controlled Phase II study (501-CL-003) in subjects with chronic ITP who were refractory to, or relapsed after, at least one prior ITP therapy, E5501 (once-daily for 28 days) was well tolerated and, at higher dose, was effective at increasing platelet counts. Here, 53 of 57 subjects who completed 28 days of study treatment (placebo, 2.5, 5, 10 or 20 mg E5501) in 501-CL-003 were treated with E5501 in a 6-month extension study, 501-CL-004. Subjects classified as responders in 501-CL-003 (i.e. those whose platelet count had risen by a minimum of 20 ×109/L above baseline to ≥50 ×109/L at Day 28) continued to receive their original E5501 blinded dose when they entered 501-CL-004. Subjects who were nonresponders in 501-CL-003 initially received open-label E5501 10 mg once-daily. E5501 dose was titrated upwards in an open-label fashion in 10 mg increments every 14 days depending on subject response (to a maximum of 40 mg once-daily for nonresponders and blinded dose plus 20 mg once-daily for responders). Additionally, concomitant medications were reduced according to subject response. Effectiveness analyses included only the 53 subjects in 501-CL-004. Safety analyses were performed on combined data from 501-CL-003 and 501-CL-004 (n=64). Subjects were regarded as responders in 501-CL-004 if their platelet count was ≥50 ×109/L and had risen by a minimum of 20 ×109/L above baseline (from 501-CL-003). Subjects had an overall platelet response if, in the absence of rescue medication, they were responders for 75% of the time over the last 14 weeks of the 24 week study (‘durable platelet response’) or were responders on any 4 consecutive weeks (‘transient platelet response’). E5501 showed effectiveness as measured by durable and overall platelet response. The durable platelet response rate was 52.8% for all subjects, 72% for subjects who were responders in 501-CL-003, and 35.7% for subjects who had been nonresponders. The overall platelet response rate was 75.5% for all subjects, 88% for responders, and almost two thirds (64.3%) for nonresponders. Following treatment with E5501, median platelet counts were maintained for the duration of treatment (Figure 1). E5501 also showed effectiveness as measured by a reduction or withdrawal of concomitant steroid medications. Among subjects using steroids, 54.2% decreased their use by >50%, including 33.3% who discontinued their use permanently. Twenty of 25 (80%) responders from 501-CL-003 maintained a platelet response throughout 501-CL-004 without requiring an upward dose titration. Twenty-one of 28 nonresponders required, but only 10 subjects received, an upward dose titration; of these, 7 (70%) achieved an overall response. E5501 was well tolerated, with a favorable safety profile. All 64 subjects (100%) experienced one or more treatment-emergent adverse events (TEAEs); most were mild, transient, and resolved completely. The most common TEAEs were fatigue (37.5%), headache (32.8%) and epistaxis (25%). TEAEs leading to study discontinuation were reported in 10/64 (15.6%) subjects. Serious TEAEs were reported in 12/64 (18.8%) subjects (Table 1). Of these, only 4 (6.3%) were considered by the investigators to be treatment-related. Forty-three of 64 subjects (67.2%) reported a bleeding event; 3 had a clinically significant grade 3 bleed (epistaxis, hemorrhagic diathesis or intracranial bleed) and 1 had a grade 4 GI bleed related to hemorrhagic gastritis. None were considered to be related to study drug. All other bleeding events were grades 1 or 2. Nine subjects met the criteria for recurrence of thrombocytopenia, defined as a platelet count that decreased to <10 ×109/L upon discontinuation of E5501. Four of these were deemed serious; all 4 recovered. Thromboembolic events were reported in 4 of 64 subjects (6.3%). One had a grade 3 deep vein thrombosis, 1 had a grade 3 stroke, and 1 had TIA and MI (Day 20) and a grade 4 retinal artery occlusion (14 days posttreatment). All 3 of these subjects had multiple risk factors for thrombosis. The fourth subject had grade 1 superficial thrombophlebitis. In conclusion, results from this 6-month extension study are supportive of the long-term efficacy and safety of E5501 in adults with difficult-to-manage, relapsed or refractory ITP.
Bussel:Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; GlaxoSmithKline: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sysmex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Consultancy. Zhang:Eisai: Employment. Tang:Eisai: Employment. McIntosh:Eisai: Employment. Kuter:Amgen: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; ONO: Consultancy; Shionogi: Consultancy, Research Funding; Pfizer: Consultancy; Protalix: Consultancy, Research Funding; Risk Managment Foundation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal