Abstract
Abstract 352
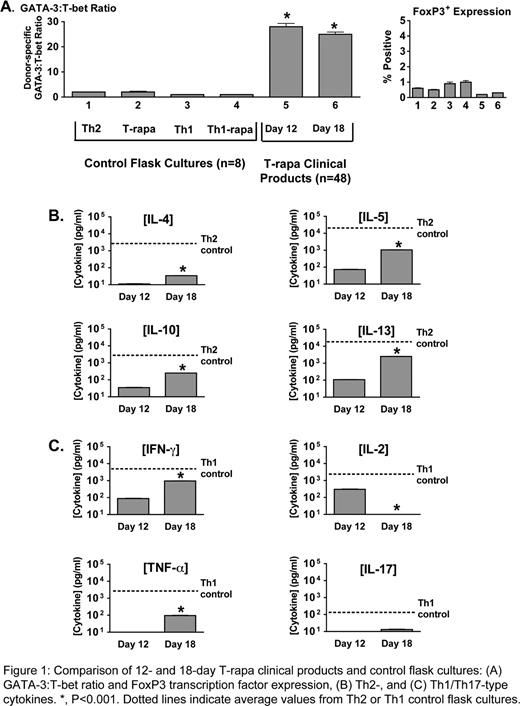
Ex-vivo expansion of murine donor CD4+ T cells using co-stimulation, IL-4, and rapamycin generated a T cell population (T-rapa cells) that beneficially modulated GVHD, graft rejection, and GVT effects. We thus conducted a clinical trial to evaluate T-rapa cell infusion after HLA-matched sibling allogeneic HCT. In one trial arm, T-rapa cell infusion (2.5 × 107 cells/kg; d 14 post-HCT) safely accelerated alloengraftment after low-intensity host conditioning, as evidenced by: conversion of mixed chimerism to predominant donor chimerism in the majority of patients by d 100 post-HCT and a low rate of grade II to IV acute GVHD (10%; 4/40 cases). This abstract characterizes the T-rapa clinical products (n=48), particularly with respect to the balance of Th1, Th2, and Treg cells and the magnitude of effector function (cytokine secretion); this latter aspect is relevant because in animal models, T cells of limited differentiation mediate increased in vivo effects upon adoptive transfer. Transplant donors underwent steady-state leukapheresis. CD4+ T cells were then purified (Miltenyi.. CliniMACS) and expanded in Lifecell.. bags for 12 days using co-stimulation (anti-CD3, anti-CD28 coated magnetic beads) and media containing rhIL-2, rhIL-4, and rapamycin. T-rapa products contained minimal cells of naive phenotype (CD45RA+ cells: 2 ± 0.4%) and were comprised of both central memory cells (CCR7+: 28 ± 2%) and effector memory cells (CCR7−: 67 ± 2%). Phenotyping assays were performed on T-rapa clinical products at d 12 of culture (time of cell product infusion); in addition, to assess phenotype stability, assays were performed after an additional 6 days of culture after co-stimulation without IL-4 and rapamycin (“day 18”). For comparison, four separate CD4+ T cell culture conditions were established from each of n=8 normal donors. For these control cultures, CD4+ T cells were co-stimulated and propagated for 12 days in tissue culture flasks using: (1) IL-2, IL-4 (“Th2”); (2) IL-2, IL-4 plus rapamycin (“T-rapa”); (3) IL-2, IFN-a (“Th1”); and (4) IL-2, IFN-a plus rapamycin (“Th1-rapa”). Phenotyping results are shown in Fig. 1. In the four flask cultures, there was modest skewing of the Th2/Th1 balance, as indicated by relatively comparable expression of the Th2 transcription factor GATA-3 and the Th1 transcription factor T-bet by intra-cellular flow cytometry (Fig. 1A). In marked contrast, day 12 T-rapa clinical products expressed a highly polarized Th2/Th1 balance (GATA-3/T-bet ratio of 28 ± 9 [mean ± SEM]); this Th2/Th1 balance was relatively stable after additional culture without IL-4 and rapamycin (“Day 18”). The median frequency of transcription factor expression was 11.5% for GATA-3 (range: 3–37%), 5.1% for T-bet (range: 0–18%), and < 1% expression of the Treg transcription factor FoxP3 (range: 0–0.7%). T-rapa clinical products were evaluated for cytokine secretion in response to co-stimulation at day 12 and day 18 of culture; supernatants were tested for Th2 cytokines (Fig. 1B) and Th1/Th17 cytokines (Fig. 1C). Day 12 T-rapa clinical products secreted each Th2 cytokine measured. The magnitude (pg/ml) of T-rapa cell Th2 cytokine secretion was approximately 2-log reduced relative to control Th2 cells; day 18 T-rapa cell secretion of Th2 cytokines was increased relative to day 12 values, but still reduced relative to control Th2 cells. Day 12 T-rapa clinical products did not secrete IL-17 and secreted low levels of IFN-g, IL-2, and TNF-a (1-3 log reduced relative to Th1 control cells). Day 18 T-rapa cell secretion of IFN-g and TNF-a was increased relative to day 12 values, but still reduced relative to control Th1 cells. Remarkably, day 18 T-rapa cell secretion of IL-2 was greatly diminished relative to day 12 values, and IL-17 secretion remained at minimal levels. In conclusion, T-rapa cell clinical products are comprised of a balance of Th2 and Th1 effector CD4+ T cells, with minimal contamination from Treg or Th17 cells. The T-rapa cell clinical products possessed limited differentiation plasticity and secreted low levels of Th2 and Th1 cytokines. Adoptive transfer of a balance of minimally differentiated and fixed polarity donor Th2/Th1 cells represents a novel approach to safely accelerate alloengraftment after low-intensity conditioning.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal