Abstract
Abstract 3410
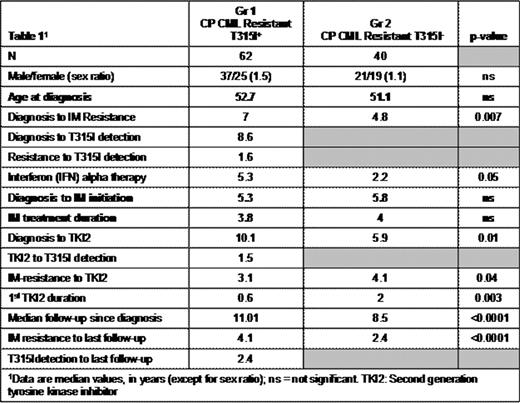
The ABL T315I mutation confers resistance to all approved tyrosine kinase inhibitors (TKIs) for the treatment of CML and Ph1+ ALL. The survival of patients harboring a T315I mutation, associated or not with other factors, is dependent on disease phase at the time of mutation detection. In vitro, in cell lines, this mutation alters kinase function and phosphorylation activity that increases oncogenicity (Skaggs et al., PNAS 2006). Using a matched-pair analysis, we aim to confirm whether or not these in vitro findings correlate into the clinic and provide higher rates of progression and poorer outcome specifically among CML patients remaining in chronic phase (CP) at T315I detection. A cohort of CP CML patients at T315I detection were identified from a database of an earlier epidemiologic study (Gr 1) was compared to a single-center, matched cohort of CML patients resistant to imatinib (IM), in CP, but not harboring the T315I mutation (Gr 2). All patients had had IM. These patients were matched on the 3 following variables: i) age at diagnosis, ii) time from diagnosis to IM initiation, iii) IM duration. The general characteristics of the 2 groups are displayed in table 1.
Univariate analysis demonstrated that patients were equivalent for all factors except for the intervals from diagnosis to IM resistance and from diagnosis to TKI2 initiation that were significantly longer in Gr 1. Sixty-two percent of the patients in Gr 1 and 100% in Gr 2 took a TKI2. The duration of treatment with a 1st TKI2 was significantly longer in Gr 2, as a significant proportion of IM-resistant patients might respond to these agents. The cumulative incidence of switches for TKI2 was similar between the 2 groups (p=0.33). In a multivariate analysis the presence of the T315I mutation had a highly significant negative impact on overall survival (OS) from any time-point (HR since IM resistance=21.6, [5.4-87.3], p<0.0001) and on progression-free survival (PFS) (HR from IM resistance=4.4, [1.9-10.1], p=0.0005). IFN treatment prior to IM (64.5% in Gr 1, 62% in Gr 2), favourably influenced the outcome of OS (HR=0.19 [0.06-0.55], p=0.002). The duration of IM treatment also had a favourable influence on OS and PFS (HR=0.19 [0.06-0.55], p=0.002 for OS, HR=0.66 [0.49-0.9], p=0.08 for PFS). A short time-lapse between CML diagnosis to IM initiation had a favourable impact (HR=1.2 [1.04-1.35], p=0.011). When comparing OS from IM resistance between Gr 1 and 2, the presence of the T315I mutation resulted in significantly poorer survival rates when compared to those in Gr 2 (median OS = 47 months for Gr 1 and not reached for Gr 2, p<0.0001, Figure 1a). Similarly, the PFS rates from the onset of IM-resistance were significantly better in Gr 2 (p=0.002) (Figure 1b).
The same patterns were observed for OS and PFS from CML diagnosis (p<0.0001 and p=0.0158, respectively), from IM start (p<0.0001 and p<0.0001), and from 1st TKI2 start (p=0.0003 and p=0.037). Even if mutations other than T315I were detected in patients from Gr 2, the OS and PFS from IM resistance of Gr 2-mutated [16/40, 40%, 1 P-Loop mutation (G250E), 1 F317L, and other] and unmutated patients were much better than that of Gr 1 (p<0.0001 for both). Other treatments, excluding allogeneic stem cell transplantation, had no impact on improving OS or PFS and were not significant in the multivariate analysis.
In conclusion, despite the limitations of this matched-pair analysis, the results strongly suggest that survival is negatively affected by the presence of the T315I mutation in CP CML patients resistant to IM, and appears to confirm in vitro observations.
Hochhaus:Ariad: Consultancy, Membership on an entity's Board of Directors or advisory committees. Peter:Merck: Employment. Zhou:Pfizer: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal