Abstract
Abstract 3398
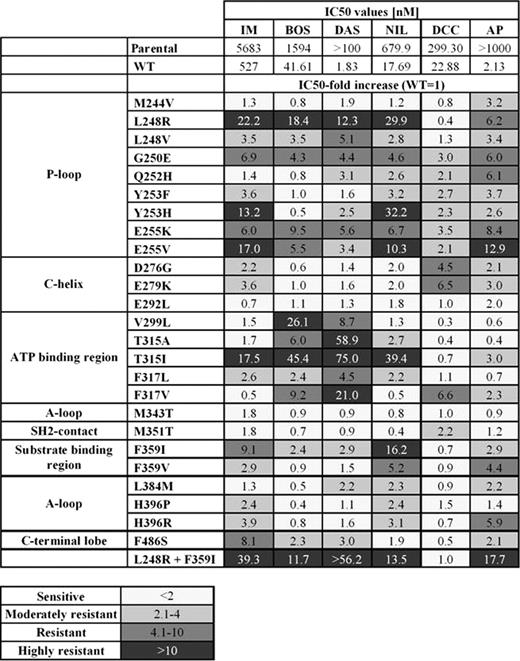
Chronic Myeloid Leukemia (CML) treatment was radically modified by the discovery of imatinib (IM), a selective inhibitor of the fusion kinase Bcr-Abl. Second generation ATP-competitive tyrosine kinase inhibitors (TKIs) bosutinib (BOS), nilotinib (NIL) and dasatinib (DAS) further improved CML therapy. However, resistance to TKIs occurs in a variable proportion of patients; it can arise from different mechanisms but in the majority of cases it is due to Bcr-Abl point mutations that alter directly or indirectly the drug-protein binding. Over 70 mutations have been described in patients, with T315I showing resistance to IM, NIL, BOS and DAS. Here we report the discovery of a new P-loop mutant (L248R) that is highly resistant to all currently available inhibitors. L248R was identified in a lymphoid Blast Crisis CML patient. The patient initially presented with an IM-resistant F359I mutation. A cytogenetic and molecular response was obtained with BOS, but after 1 year haematological relapse developed; at this point a F359I/L248R clone was identified. L248R was previously reported only in an in vitro during a mutagenesis screen involving IM (Bradeen et al. 2006) and in a study with the T315I inhibitor SGX393 (O'Hare et al. 2008). L248R was never isolated from a clinical sample. Activity profile of BOS, IM, DAS and NIL against L248R, F359I and F359I/L248R was performed (Table). Stable transfectant Ba/F3 cells were generated and the TKIs anti-proliferative activity was determined. The relative IC50 increase over wild type Bcr-Abl (Relative Resistance, RR) was calculated. We classified RR values in four categories: sensitive (RR≤ 2), moderately resistant (RR between 2.1 and 4), resistant (RR between 4.1 and 10) or highly resistant (RR>10). In all cases a RR >10 was obtained, for L248R. Recently new compounds were developed to overcome resistance generated by the T315I mutant. Among them AP-24534 (AP), a panBcr-Abl inhibitor (O'Hare et al. 2009) and the switch pocket inhibitor DCC-2036 (DCC) were reported as potently active against the T315I mutation. Both compounds showed activity against L248R (RR 6.2 and 0.4), although the activity against the double mutant F359I/L248R was reduced especially for AP (RR 17.7 and 1.0). The activity profile of AP and DCC against L248R and a panel of 26 mutated forms of Bcr/Abl covering the most common mutations is also presented (Table). Activity of BOS, IM, DAS and NIL is also reported for comparison. According to our data, only mutation E255V is classified as highly resistant to AP, in addition to F359I/L248R: 6/26 mutations are considered “resistant” to AP (L248R, G250E, G252H, E255K, F359V, H396R) and 3/26 to DCC (D276G, E279K, F317V). The huge difference in the IC50 values for AP between Bcr/Abl wild type (2.1 nM) and parental Ba/F3 cells (>1000 nM) could render some of the mutants with high RR values to AP still sensitive to this inhibitor. It is also important to note that, according to our data, every mutation analysed shows sensitivity to at least one of the tested TKIs. In conclusion we describe a novel mutation that is highly resistant to the commonly used Bcr-Abl TKIs but which is inhibited by AP and DCC. Modelling data on the L248R mutant in complex with different TKIs will also be presented
IC 50 values are based on tritiated thymidine incorporation assay. Results are an average of at least 3 independent experiments
Wise:Deciphera Pharmaceuticals: Employment. Flynn:Deciphera Pharmaceuticals: Employment. Gambacorti-Passerini:Pfizer pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal