Abstract
Abstract 3249
L-asparaginase (ASP) is a universal component of treatment for childhood acute lymphoblastic leukemia (ALL). It is associated with multiple toxicities, including allergy, pancreatitis, and thrombosis. It is not thought to have a significant direct myelosuppressive effect, but its impact on the ability to tolerate other chemotherapeutic agents has not been well-characterized.
Between 2005–2010, 61 patients (pts) were diagnosed with Standard Risk (SR) ALL at DFCI/Children's Hospital Boston and treated on DFCI ALL Consortium Protocol 05-01. SR pts met all of the following criteria: 1 to <10 years of age; highest pretreatment WBC < 50,000/mm3; absence of central nervous system leukemia (ie, CNS-1 or -2, only); absence of T-cell markers; absence of t(9;22), MLL gene rearrangements, and hypodiploidy; and low minimal residual disease (MRD) levels at the end of the first 4 weeks of treatment. Beginning at week 7, all SR pts began a 30-week (wk) Consolidation phase, consisting of every 21-day cycles of vincristine (day 1), dexamethasone (days 1–5), 6-mercaptopurine (6MP) (days 1–14), and methotrexate (MTX) (days 1, 8 and 15), and also including 30 consecutive wks of ASP (either weekly IM E.coli ASP 25000 IU/m2 or every 2-wk IV PEG ASP 2500 IU/m2). The Continuation phase followed Consolidation, and consisted of identical systemic and intrathecal (IT) chemotherapy, except without any ASP. Starting dose of 6MP was 50 mg/m2/day and of MTX was 30 mg/m2/dose. Doses were adjusted every 21 days to maintain absolute phagocyte (APC) nadirs > 500/mm3 and platelet nadirs > 75,000. Chemotherapy cycles were delayed until APC was ≥ 1000/mm3 or platelets were ≥ 100,000. Medical records of the 61 SR pts were reviewed to ascertain the dose modifications of MTX and 6MP made due to low or high blood counts (APC and/or platelets) and/or delays in starting cycles (defined as ≥25 day interval between cycles). 27 pts were randomized to receive IM E. coli ASP, 29 to IV PEG ASP, and 5 declined randomization (directly assigned to IM E.coli ASP). A total of 1780 chemotherapy cycles were evaluated longitudinally using generalized estimating equations, 498 during Consolidation (with ASP) and 1282 during Continuation (No ASP).
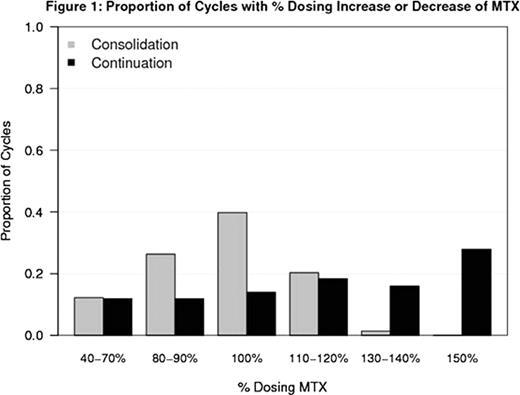
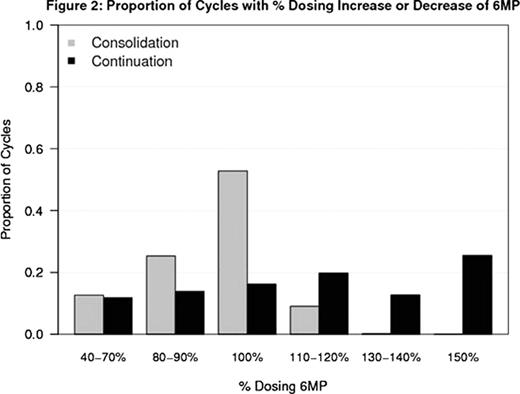
Median age was 3.9 years and 57% of pts were female. There was a higher proportion of cycles modified for low blood counts (ie, dose reduction of MTX and/or 6MP) during Consolidation (ASP given) compared to Continuation (No ASP) (24% vs 8%, Odds Ratio (OR) = 2.35 [95% CI 1.41, 3.94]; p < 0.01). There was a lower proportion of cycles modified due to high blood counts (ie, dose escalation of MTX and/or 6MP) during Consolidation (ASP given) compared to Continuation (No ASP) (20% vs 33%, OR =0.20 [95% CI 0.13, 0.30]; p < 0.01). Figures 1 and 2 summarize the dosing of MTX and 6-MP during Consolidation and Continuation. During Consolidation, the proportion of cycles in which pts received escalated doses of MTX (above the starting dose) was 21% compared to 62% during Continuation. Similarly, the proportion of cycles in which pts received escalated doses of 6-MP (above starting dose) was 10% in Consolidation compared to 59% in Continuation. 17% of Consolidation cycles were delayed because starting criteria were not met compared to 9% of Continuation cycles when pts were not receiving ASP (OR =2.08, 95% CI 1.18, 3.64; p=0.01). No difference in delays or dose modification for counts was detected between ASP randomization arms in either the Consolidation or Continuation phases.
Proportion of Cycles with % Dosing Increase or Decrease of MTX
Proportion of Cycles with % Dosing Increase or Decrease of MTX
Proportion of Cycles with % Dosing Increase or Decrease of 6MP
Proportion of Cycles with % Dosing Increase or Decrease of 6MP
During Consolidation, when SR pts received ASP, blood counts were lower than during Continuation (no ASP), despite an otherwise identical schedule of other systemic and IT agents during these two phases. Because of low blood counts, pts received lower doses of MTX and 6MP when they were also receiving ASP, and delays in beginning chemotherapy cycles were more common. This suggests that ASP has a myelosuppressive effect, either direct (due to ASP) or secondary to ASP-induced changes in the levels or metabolism of other chemotherapeutic agents; further investigation is necessary to elucidate the precise mechanism. The myelosuppressive effect of ASP should be taken into consideration when designing regimens for pts with ALL which combine ASP with other agents to avoid excessive dose reductions and/or treatment delays.
Sallan:Enzon Inc.: Honoraria, Research Funding. Silverman:EUSA Pharmaceuticals: Consultancy, Honoraria; Enzon Pharmaceuticals: Consultancy, Honoraria.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal