Abstract
Abstract 3132
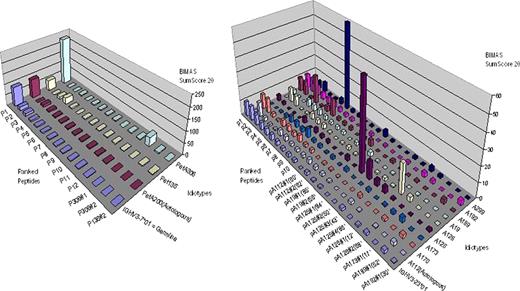
The idiotype (Id) of malignant lymphomas is a tumor-specific antigen and has been evaluated for active immunotherapy predominantly in follicular lymphoma (FL). We have previously described “reverse immunology” evidence that HLA class I-dependent and hence presumably T-cell mediated selection pressure restricts the growth of malignant FL clones with comparatively highly immunogenic Ids (Strothmeyer et al., Blood, epub 2010). This effect was mostly attributable to the Id CDRs. We therefore hypothesized that somatic hypermutation, a constitutively active process in FL, is the mechanism that creates immunogenic Id epitopes and enables T-cell mediated immunosurveillance. To test this hypothesis, we analyzed Id of FL that utilized the same Vh gene but arose in individuals with different HLA types (IGHV3-23*01, n=9 patients: IGHV3-7*01, n=3). For every represented HLA type, an “immunogenicity landscape” mapping was performed by plotting the ten most immunogenic peptides of the germ-line Vh sequence in comparison to FL Id peptides in the order of their HLA binding strength as predicted by the BIMAS algorithm for the respective patient. Neither changes in the order of peptides nor major differences in their binding scores were observed. Parallel plotting of Vh-related Ids from different HLA types revealed novel peptides with higher binding scores within and in addition to the ten highest ranking epitopes (Figures). Comparisons of these peptides with the germ-line and the index patient's sequence directly demonstrated that somatic hypermutation creates peptides that would have strongly bound to HLA type of the index patient but were immunologically neutral in the patient of origin. Occurrence of such highly immunogenic peptides by somatic hypermutation would hence not be tolerated by an FL patient's immune system. To explore whether this mechanism also operates in other lymphoma types, we analyzed predicted HLA-binding Id peptides from mantle cell (n=20) and marginal zone (n=12) lymphomas. Like in FL, the median sum of the 20 peptides with the highest BIMAS scores were to various degrees less immunogenic on the autologous HLA type than on allogeneic HLA types (ratio of autologous to allogeneic BIMAS sum scores: FL: 0.63; MCL: 0.78; MZL: 0.87). In subanalyses of the CDR, these differences were observed only in FL and MCL but not in MZL (CDR1: FL 0.40, MCL 0.55. MZL 1.39; CDR2: FL 0.25, MCL 0.36; MZL 1.54: CDR3: FL 0.43, MCL 0.53, MZL 1.28).
To investigate whether HLA-dependent immunosurveillance acts on normal B-cell receptors, we compared BIMAS sum scores of 96 non-clonal Ids present in diagnostic biopsies of 29 FL and MCL patients. These Ids represent bona fide contaminating, healthy B cells. No significant differential immunogenicity existed for the entire Vh sequences or individual CDR regions, regardless of their mutational status. We therefore randomly sequenced B-cell receptors from two mouse strains with an identical B6 genetic background but a congenic H-2 complex (C57BL/6ByJ: H-2b; 422 sequences; B6-H2d/bByJ: H-2d; 412 sequences). In these SPF-bred mice, 61% and 71% of sequences, respectively, were unmutated. Significantly higher immunogenicity was observed in the FR1-3 regions of C57BL/6ByJ compared to B6-H2d/bByJ Ids on H-2kd (p=0.04). Also, several Vh genes were significantly (Fisher's exact test) less frequent in mice expressing the MHC type with stronger binding to Id-derived peptides of the corresponding germ-line Vh sequence. Overall, the ratio of predicted MHC binding strength of the 20 highest scoring peptides from 59 represented germ-line Vh genes correlated to the relative frequency of representation of these genes in the peripheral B-cell repertoire (p=0.016, Pearson correlation). Collectively, these results indicate that somatic hypermutation is the predominant mechanism that enables T-cell mediated control of malignant B-cell clones in FL but possibly also MCL on the basis of the HLA binding of Id-derived peptides. In addition, HLA binding strength of germ-line-encoded Vh peptides appears to exert a subtle modulation of the primary B cell repertoire by MHC class I-restricted T cells. These findings strengthen the theoretical basis of active Id-directed immunotherapy of B-cell lymphomas with mutated antigen receptors. In addition, they may also lead to a novel explanation for the association of HLA type with aberrant functions of the nonmalignant immune system.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal