Abstract
Abstract 3053
Patients with smoldering multiple myeloma (SMM) have a higher chance of progression to active MM than patients with monoclonal gammopathy of undetermined significance (MGUS). Since active MM remains incurable and since patients with SMM can have a long time to requiring treatment, observation remains an option for these patients. Bisphosphonates can prevent the bone complications of myeloma. The immunomodulatory (IMiD) drugs are well-tolerated and have documented anti-tumor activity in active MM. We hypothesized that treatment with the IMiD THAL and a bisphosphonate (ZLD) would prolong the time to progression (TTP) over the control arm of ZLD alone. This is the first report of this phase III trial of THAL/ZLD vs ZLD alone for patients with untreated SMM.
The primary goal of this trial was to compare the TTP between patients treated with THAL/ZLD versus ZLD alone in asymptomatic MM. Secondary goals were progression rate at one year, response rate, duration of response, time to next therapy, and toxicity.
Patients were required to have measurable disease as defined by either a serum monoclonal protein >1.0 g by protein electrophoresis or nephelometry or >200 mg of monoclonal protein in the urine on 24 hour electrophoresis or a measurable soft tissue plasmacytoma; >10% plasma cells as measured on the bone marrow aspirate, bone marrow biopsy, or labeling index; absolute neutrophil count >1500/μL; platelet count >100,000/μL; creatinine <2.0 mg/dL, and a performance status of 0, 1, or 2. Patients could not have symptomatic MM that required chemotherapy. The statistical plan was to accrue 120 eligible patients (60 per arm) over 4 years and after a minimum follow-up of 12 months the study would provide >90% power at a type I error rate of 0.05 to detect an increase in the median TTP from 12 months (ZLD) to 24 months (THAL/ZLD), and >80% power to detect an increase in median TTP from 12 to 21 months).
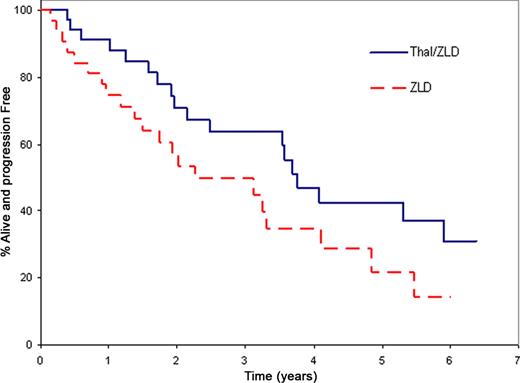
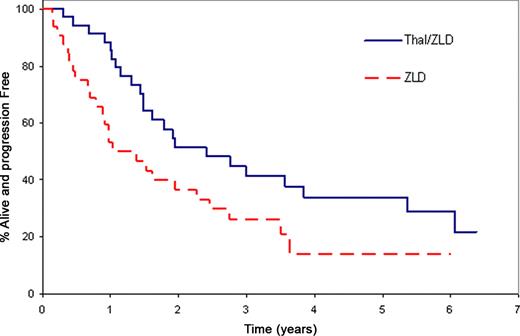
The study was activated in July 2003 and closed March 2009 due to slow accrual. Sixty-eight patients (35 Thal/ZLD; 33 ZLD) with a median age 63 years (range, 47–84) were randomized. The median TTP for Thal/ZLD versus ZLD was 2.4 years versus 1.2 years, respectively, P=0.02 one-sided log-rank. After adjusting for pre-specified stratification factors, the hazard ratio for TTP was 2.2 (one-sided p value = 0.01, univariate stratified Cox PH model) for ZLD compared to Thal/ZLD. 89% of patients on the Thal/ZLD arm were progression-free survival (PFS) at one year compared to 55% on ZLD alone (one-sided p<0.001, chi-square). Similar results were obtained (Table and Figures) when the CRAB (calcium, renal, anemia, bone) criteria were applied. Confirmed response was evaluated using the first 12 months of treatment. In the Thal/ZLD arm the response rate was 31% with a median duration of response of 5.1 years (95% CI: 1.9 - NA). There were no confirmed responses in the ZLD alone arm. The median overall survival has not been reached for either arm. In regards to toxicity, no grade 5 adverse events (AEs) have been reported. Thirty patients have reported grade 3+ AEs (17 Thal/ZLD; 13 ZLD, Fisher's exact p-value 0.47). Eight patients have reported grade 4 adverse events (5 Thal/ZLD; 3 ZLD, Fisher's exact p-value 0.71). Overall, only one grade 4 event was felt to be at least possibly related to study treatment – grade 4 neutropenia in Thal/ZLD.
While the trial did not meet its planned accrual goals, there was a significant improvement in improved in outcomes in the Thal/ZLD arm compared to control (ZLD). Thal/ZLD produces anti-tumor responses that are quite durable with the median response duration of over 5 years. ZLD alone did not produce any anti-tumor responses. This study indicates that a non-chemotherapy approach can be effective in SMM and may be a useful strategy to test in future studies.
TTP & PFS Estimates for Protocol and CRAB Progression Criteria
| Criteria . | Thal/ZLD (n=35) . | ZLD (n=33) . | p-value (unadjusted, log-rank, one-sided) . | p-value (adjusted for stratification factors using a univariate Cox model, one-sided) . |
|---|---|---|---|---|
| TTP Protocol | 2.4 years | 1.2 years | 0.02 | 0.01 |
| # of Events | 21 | 24 | ||
| TTP CRAB | 4.1 years | 3.1 years | 0.03 | 0.02 |
| # of Events | 15 | 20 | ||
| PFS Protocol | 2.4 years | 1.2 years | 0.02 | 0.005 |
| # of Events | 23 | 25 | ||
| PFS CRAB | 3.8 years | 2.3 years | 0.03 | 0.008 |
| # of Events | 18 | 22 |
| Criteria . | Thal/ZLD (n=35) . | ZLD (n=33) . | p-value (unadjusted, log-rank, one-sided) . | p-value (adjusted for stratification factors using a univariate Cox model, one-sided) . |
|---|---|---|---|---|
| TTP Protocol | 2.4 years | 1.2 years | 0.02 | 0.01 |
| # of Events | 21 | 24 | ||
| TTP CRAB | 4.1 years | 3.1 years | 0.03 | 0.02 |
| # of Events | 15 | 20 | ||
| PFS Protocol | 2.4 years | 1.2 years | 0.02 | 0.005 |
| # of Events | 23 | 25 | ||
| PFS CRAB | 3.8 years | 2.3 years | 0.03 | 0.008 |
| # of Events | 18 | 22 |
Off Label Use: Thalidomide for smoldering myeloma. Lacy:Celgene: Research Funding. Dispenzieri:Celgene: Honoraria, Research Funding; Binding Site: Honoraria. Kumar:Celgene: Consultancy, Research Funding; Millennium: Research Funding; Merck: Consultancy, Research Funding; Novartis: Research Funding; Genzyme: Consultancy, Research Funding; Cephalon: Research Funding. Gertz:Celgene: Honoraria; Millennium: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal