Abstract
Abstract 3026
UPFRONT is an ongoing US community-based phase 3b study designed to compare the safety and efficacy of three bortezomib (Velcade®, Vc)-based regimens, Vc-dexamethasone (VcD), Vc-thalidomide-dexamethasone (VcTD), and Vc-melphalan-prednisone (VcMP), followed by Vc maintenance therapy, in previously untreated multiple myeloma (MM) patients who were ineligible for high-dose therapy and autologous stem cell transplantation (HDT-SCT). Efficacy data have been presented elsewhere; here we present patient-reported quality of life (QoL) data after 300 patients had the opportunity to undergo the entire 13-cycle treatment period (8 Vc-based induction cycles and 5 Vc maintenance cycles).
Patients with symptomatic, measurable MM were randomized (1:1:1) to receive 49 weeks of therapy: 24 weeks (eight 21-day cycles) of induction with VcD, VcTD, or VcMP (VcD: Vc 1.3 mg/m2, days 1, 4, 8, 11; D 20 mg, days 1, 2, 4, 5, 8, 9, 11, 12 [cycles 1–4]), days 1, 2, 4, 5 [cycles 5–8]); VcTD: Vc 1.3 mg/m2, days 1, 4, 8, 11; T 100 mg/d, d1–21; D 20 mg, days 1, 2, 4, 5, 8, 9, 11, 12 [cycles 1–4]), days 1, 2, 4, 5 [cycles 5–8]); VcMP: Vc 1.3 mg/m2, days 1, 4, 8, 11; M 9 mg/m2, and P 60 mg/m2, day 1–4, every other cycle), followed by 25 weeks (five 35-day cycles) of maintenance with Vc alone (1.6 mg/m2, days 1, 8, 15, 22). Patient-reported QoL was recorded using the EORTC QLQ-C30 questionnaire, which assesses global health status, physical, role, cognitive, emotional, and social functions, fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties. Patients completed the questionnaire prior to dosing on cycle 1, day 1 (baseline), prior to dosing on day 1 of every odd cycle, at the end of treatment visit, and every 12 weeks thereafter. Here we present updated study results after 300 patients had the opportunity to undergo the entire 13-cycle treatment period, focusing on change in global health status score over time; no adjustment was made for missing cases or patient deaths.
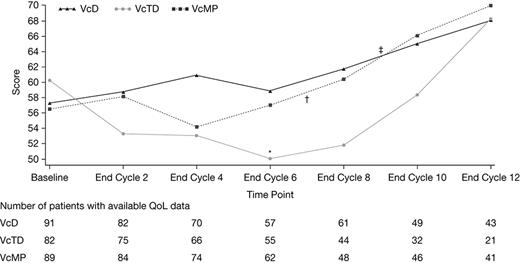
Patient baseline characteristics were well balanced across the three treatment arms. Patients in the VcD, VcTD, and VcMP arms had median ages of 73.5, 73.0, and 72.0 years, respectively; 85%, 64%, and 74% had ISS stage II/III; 22%, 27%, and 28% were non-Caucasian; and 51%/25%/24%, 55%/30%/14%, and 62%/24%/15% had Charlson comorbidity index 0/1/≥2 in the VcD, VcTD, and VcMP arms, respectively. Patients received a median of 9 (VcD), 6 (VcTD), and 7 (VcMP) treatment cycles (induction + maintenance); 56%, 33%, and 43% of patients, respectively, received Vc maintenance. In the VcD, VcTD, and VcMP arms, Vc dose intensity (mean ratio of doses received/doses planned) was 76%, 63%, and 69% during induction, and 73%, 77%, and 85% during maintenance, respectively. After 13 treatment cycles, the rates of grade ≥3 adverse events (AEs) were 74%, 86%, and 80% in the VcD, VcTD, and VcMP arms. The incidence of serious AEs was highest in the VcTD arm (61%, vs 57% and 51% with VcD and VcMP), as was the rate of study drug discontinuation due to AEs (41%, vs 29% and 35% with VcD and VcMP). Global health status score by cycle is shown in the Figure, with number of patients with available QoL data in each arm indicated below. In all three arms, for those patients with available QoL data, global health status score at the end of cycle 12 was greater than at baseline and at the end of cycle 8 (end of Vc-based induction therapy). In the induction phase, global health status score remained stable in the VcD arm and transiently decreased in the VcMP arm at cycle 4, before increasing to above baseline levels. A similar trend was seen for the VcTD arm; global health status score started decreasing at cycle 2 but took slightly longer to increase, possibly due to the increased incidence of AEs in the VcTD arm. Similar trends were seen for other EORTC QLQ-C30 function and symptom scores.
Although there was some variability during the study, by the end of the treatment period patients who received one of the three Vc-based regimens reported improvements in QoL compared with baseline values. The study is ongoing and patients continue to be followed for assessment of patient-reported QoL and long-term outcomes. Updated QoL data, including the correlation between change in QoL and best response achieved, will be presented.
Change in global health status score by cycle in the VcD, VcTD, and VcMP arms; *†‡ indicate median number of cycles for each arm
Change in global health status score by cycle in the VcD, VcTD, and VcMP arms; *†‡ indicate median number of cycles for each arm
Niesvizky:Celgene: Consultancy, Research Funding; Millennium Pharmaceuticals, Inc.: Consultancy, Research Funding; Onyx: Consultancy, Research Funding. Off Label Use: Discussion of Velcade in a novel combination in frontline myeloma is included. Flinn:Millennium Pharmaceuticals, Inc.: Research Funding. Rifkin:Millennium Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Speakers Bureau; Amgen: Speakers Bureau; Cephalon: Speakers Bureau; Dendreon: Speakers Bureau. Gabrail:Millennium Pharmaceuticals, Inc.: Research Funding. Charu:Amgen: Equity Ownership, Research Funding; Pfizer: Equity Ownership; GSK: Equity Ownership, Research Funding; Lilly: Equity Ownership, Research Funding; Millennium Pharmaceuticals, Inc.: Research Funding; Roche: Research Funding; Bristol-Myers Squibb: Equity Ownership. Neuwirth:Millennium Pharmaceuticals, Inc.: Employment. Corzo:Millennium Pharmaceuticals, Inc.: Employment. Reeves:Celgene: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal