Abstract
Abstract 2746
Newly diagnosed and relapsed acute promyelocytic leukemia (APL) patients respond to therapy with arsenic trioxide (ATO) based regimens. Significantly more patients with relapsed APL have disease recurrence after ATO based therapy than newly diagnosed cases. We undertook a series of experiments to evaluate the potential mechanisms to explain this increased recurrence rate in patients with relapsed APL.
Patients and methods: From April 2007 to March 2009 bone marrow samples from newly diagnosed and relapsed cases admitted at our center were utilized for these studies. If required the bone marrow blasts and promyelocyte component was enriched to above 90% using a lineage depletion cocktail and VarioMACS (Miltenyi Biotec, Germany). For in-vitro intracellular ATO concentration measurement, 2 × 107 cells were washed and suspended in RPMI media with 0.5 μM concentration of ATO and incubated for 24 hours. Cells were then washed, made into a pellet and solubilized with HNO3 and H2O2 and ATO content measured using an atomic absorption spectrophotometer. An in-vitro sensitivity assay of malignant cells as previously reported was standardized using an MTT assay system. The impact of co-culture of mesenchymal stromal cells (MSC) and malignant promyelocytes on ATO induced apoptosis was studied with 7AAD and Annexin staining using a flowcytometer. A gene expression array using 44k human microarray chip analysis (Agilent technologies) was done on 8 newly diagnosed and 8 relapsed cases.
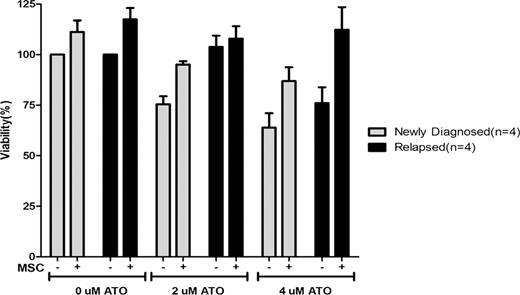
Sixty five patients were included in this study. Of these 47 (72%) were newly diagnosed and 18 (28%) were relapsed cases. On immunophenotyping, CD34 was positive (>20%) in 3.6% of newly diagnosed and 50% of relapsed cases (P=0.001). The mean MFI of the relapsed cases for expression of CD38, VLA-5 and CD13 was significantly lower in the relapsed group. The ability of both newly diagnosed and relapsed primary APL cells to concentrate ATO intracellular was not significantly different (15.2±9 nG/107 cell Vs. 16.3±9.7 nG/107 cell). Similarly the in-vitro IC-50 assay was not significantly different between the two groups (5.5±3.8 Vs. 4.7±4.5 μM). Neither of these assays correlate with clinical parameters such as relapse, event free (EFS) or overall survival (OS). Evaluation of the impact of MSC on ATO induced apoptosis demonstrates a protective effect in newly diagnosed and relapsed cases (Fig 1). This effect was mediated partly by the MSC conditioning media and could not be overcome by addition of VLA-4 or VLA-5 blocking antibodies (data not shown). Gene expression studies comparing the two cohorts revealed 1744 genes that were differentially expressed (>2 fold) between samples at diagnosis and at relapse. Quantitative Real-time RT-PCR using SYBR- Green detection system was done to confirm the gene expression results obtained from microarray analysis. Using ΔΔCT method the fold difference was calculated for five selected genes which validated the microarray data.
Relapsed patients have significant immuno-phenotypic differences from newly diagnosed cases. Mechanisms of resistance to ATO are probably multi-factorial but are unlikely to be related to intra-cellular ATO concentration. In-vitro IC-50 does not appear to predict clinical outcomes. Stromal interaction protects malignant promyelocytes from the apoptotic action of ATO which appears more pronounced in relapsed than in newly diagnosed cases. Further evaluation of parameters that enhance such stromal interaction and protect malignant promyelocytes from the apoptotic effect of ATO along with evaluation of dysregulated genes and pathways are required.
Effect of stromal interaction on effect of ATO on malignant promyeloctes and blasts from newly diagnosed (n=4) and relapsed patients (n=4) with APL. The bar graph is normalized such that newly diagnosed patients cells not exposed to ATO should have 100% viability after 48 hour incubation.
Effect of stromal interaction on effect of ATO on malignant promyeloctes and blasts from newly diagnosed (n=4) and relapsed patients (n=4) with APL. The bar graph is normalized such that newly diagnosed patients cells not exposed to ATO should have 100% viability after 48 hour incubation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal