Abstract
Abstract 2743
CD52 is a cell surface glycoprotein of unknown function that is expressed in B and T lymphocytes, macrophages, and monocytes, but is not expressed in normal hematopoietic stem/progenitor cells. CD52 is also expressed in chronic lymphocytic leukemia (CLL), B-cell acute lymphoblastic leukemia (ALL), and some cases of T-ALL. Alemtuzumab, a recombinant humanized monoclonal antibody, targets CD52 and is used to treat CLL. In contrast to normal hematopoietic stem/progenitor cells, CD52 expression has been described in acute myeloid leukemia (AML) and in blast crisis (BC) chronic myeloid leukemia (CML). Based on these observations we were curious whether CD52 expression distinguished normal from malignant or more mature from immature stem/progenitors cells, and whether these cells were sensitive to alemtuzumab.
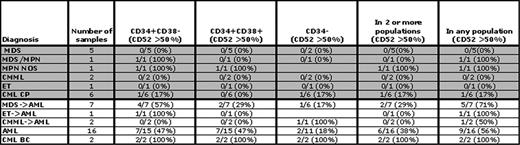
CD52 expression was examined in three blast cell populations (CD34+/CD38-, CD34+/CD38+, and CD34-) in patients with myeloid (44) and lymphoid (18) neoplasms, and normal patients (6). In normal hematopoietic cells, stems cells are enriched in the first population; more mature cells are characterized by increasing CD38 expression and loss of CD34 expression. In AML and CML leukemia stem cells may arise within either CD34+ population and possibly in the CD34- population. Relative to normal lymphocytes average CD52 expression could be characterized as low to moderate. Using an expression cutoff of > 20%, in contrast to normal patients, CD52 was detected in at least one of three blast populations in almost all patients. Using a more stringent cutoff of > 50%, CD52 was expressed in CD34+/CD38- cells in 7/11 B-ALL and 6/7 T-ALL cases and was concordantly expressed in the other two populations. Using the same criteria in myeloid malignancies (Table 1 ), expression occurred more frequently in AML, AML arising from myelodysplastic syndrome (MDS), and BC CML. In AML and AML arising from MDS, CD52 was expressed in the 34+/38- population in 7/15 cases (47%) and 4/7 cases (57%), respectively; it was expressed in both BC CML patients. In AML and BC CML patients, CD52 was expressed at similar levels in the CD34+/CD38+ fraction. No clear association between CD52 expression and cytogenetic abnormalities was found. We then examined whether CD52 expression differentiated normal from malignant blasts (CD34+/CD38- and CD34+/CD38+) in two CML myeloid BC patients. FISH and quantitative PCR demonstrated that BCR-ABL was expressed in all 4 populations, which were also morphologically distinct. Colony forming unit (CFU) assays demonstrated a significantly decreased ability to form CFU (on average 5–20 fold decrease) in CD52+/CD34+/CD38- CML cells suggesting CD52 cells may be more mature. Lastly and not previously described, we found that several BC CML cell lines express CD52, and complement-mediated cell cytotoxicity was similar in the highest expressing cell lines to that seen in EHEB (B-CLL) cells known to be targeted by alemtuzumab. Thus, alemtuzumab may have clinical efficacy in BC CML.
In conclusion, CD52 is expressed on blast populations enriched for leukemic stem cells. Whether the absence or presence of CD52 more precisely segregates a leukemia stem cell containing population currently remains unknown and requires functional testing in a murine model. Our preliminary experiments in CML suggest CD52 may not differentiate between normal and malignant stem/progenitor cells. However, CD52 expression may distinguish normal and malignant stem cell populations in cases where CD52 and CD38 are more highly expressed. The observation that CD52 expression is increased in acute vs. chronic leukemias raises the intriguing possibility that CD52, if not directly involved, may be a marker for genes or pathways contributing to the block in differentiation seen with progression to acute leukemia. Furthermore, given that CD52 expression is heterogeneous in chronic disorders, it is possible that CD52 expression within these populations may correlate with poor prognosis or impending leukemic conversion.
The proportion of patients (44) expressing CD52 at levels > 50% in 3 blast populations. Three populations were present in most, but not all patients. Gray shading indicates chronic myeloid diseases. MPN is myeloproliferative neoplasm; NOS is not otherwise specified; ET is essential thrombocythemia; CMML is chronic myelomonocytic leukemia; and an arrow represents progressed to.
Oehler:Pfizer: Research Funding. Radich:Novartis: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal