Abstract
Abstract 2692
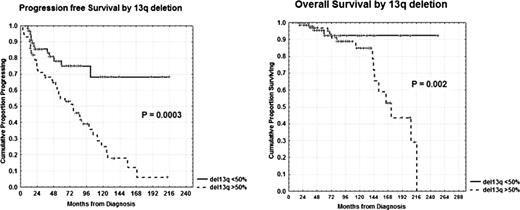
Cytogenetic aberrations are considered major prognostic indicators for predicting the survival of chronic lymphocytic leukemia (CLL) patients (pts) [Dohner et al, 2000]. Given the difficulties in obtaining abnormal metaphases in CLL, fluorescent in situ hybridization (FISH) with specific probes is generally used to detect the most frequent abnormalities. Interphase FISH (I-FISH) is a rapid and sensitive technique for analysis of chromosome aberrations in CLL, but the limited number of DNA probes available to screen B-CLL results in a high number of cases presenting normal cytogenetics. Moreover, deletion 13q14 on FISH analysis which is the most common cytogenetic abnormality in CLL is a favourable prognostic biomarker when detected as a sole abnormality, but a higher percentage of 13q- nuclei was found to be associated with significantly shorter time to treatment (P<0.001), [van Dike et al, 2010]. On this line, the primary endpoints of our research were: 1) to determine progression free survival (PFS) and overall survival (OS) within the normal FISH cytogenetics subset on the basis both of ZAP-70, CD38, CD69 expression by flow cytometry and IgVH status; 2) to correlate percentages of 13q- nuclei (<50% or ≥50%) with PFS and OS and finally 3) to confirm I-FISH as an independent prognostic factor. We investigated 400 pts, median age 65 years (range 33–89), 235 males and 165 females. With regard to modified Rai stages, 115 pts had a low stage, 266 an intermediate stage and 19 a high stage. One hundred forty-two pts (35.5%) exhibit a normal karyotype, 133 pts (33.2%) showed an isolated 13q-, 69 pts (17.3%) presented trisomy 12, 30 pts (7.5%) 11q deletion, 20 (5%) 17p deletion and 6 (1.5%) other chromosome abnormalities. Clearly, pts with intermediate/poor cytogenetic abnormalities (trisomy 12, del11q-, del17p-) showed significant shorter PFS and OS (8% vs 33% at 16 years, P<0.0001 and 41% vs 65% at 16 years, P=0.0001) in comparison with normal or del13q- pts. Therefore, in order to better define the apparently homogenous subset of the patients with normal cytogenetics, we analysed ZAP-70, CD38, CD69 and IgVH status within this subgroup. Interestingly, we observed significant longer PFS and OS for ZAP-70 negative pts (60% vs 29% at 10 years, P<0.0001 and 92% vs 23% at 16 years, P=0.0006), for CD38 negative pts (54% vs 22% at 10 years, P=0.0008 and 83% vs 0% at 14 years, P=0.03), for CD69 negative pts (60% vs 23% at 10 years, P=0.0003 and 83% vs 63% at 14 years, P=0.02) and finally also for IgVH mutated cases (64% vs 35% at 10 years, P=0.0002 and 95% vs 45% at 14 years, P=0.04). Moreover, pts with isolated 13q- in <50% of nuclei (60 pts) showed a longer PFS and OS (68% vs 6% at 16 years, P=0.0003 and 92% vs 0% at 18 years, P=0.002; Figure) compared to those with ≥50% of nuclei (72 pts). No significant correlation was found between 13q- percentages and CD38 or ZAP-70 or IgVH status, while it was found with CD69 expression (P=0.01). In multivariate analysis of PFS, FISH cytogenetics was confirmed to be an independent prognostic factor (P=0.002) together with Rai stages (P=0.005), ZAP-70 (P=0.0001), IgVH status (P=0.0002) and CD69 (P=0.007). Therefore, in the clinical practice, ZAP-70, CD38, IgVH status and CD69 are irreplaceable in order to define prognosis and treatment within the large series of CLL pts with normal cytogenetics, probably presenting abnormalities undetectable by FISH. Besides, the percentage of nuclei exhibiting 13q- has to be considered an important predictor of the outcome and the clinical implications of 13q- in CLL seem to appear more complex than originally considerated. In conclusion, a comprehensive scoring prognostic system based on a combination of clinical, genetic, phenotypic parameters and IgVH status improves the separation of prognostic subgroups in CLL already early in the course of the disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal