Abstract
Abstract 2460
Anti-CD20 (rituximab) based immuno-chemotherapies are considered standard of care in CLL. However, rituximab as single agent has limited activity in CLL. In addition, Rituximab does not appear to decisively alter the clinical outcome for 17p- / TP53 mutated CLL. CD20 is expressed at low levels in CLL and alternative targets for antibody-based treatment have been explored. CD37, a member of the tetraspanin superfamily, is a heavily glycosylated cell surface molecule with four transmembrane domains and two extracellular loops. CD37 is almost exclusively expressed on mature B cells, with highest expression levels on peripheral blood B cells, reduced levels on plasma cells and non- detectable levels on CD 10+ precursor B cells in the bone marrow. Lower level expression of CD37 has also been reported on T cells, granulocytes, and monocytes.
In an effort to assess the activity of two CD37 antibodies in CLL, we studied B cell depletion / apoptosis in a set of CLL patients in vitro. MAb 37.1 is a chimeric IgG1-type of antibody which is Fc-engineered to improve ADCC activity. MAb 37.2 is a humanized version of mAb 37.1.
CLL samples were characterized with respect to genetics (genomic aberrations, TP53 mutation, IGHV mutation status), as well as clinical course and immunophenotype. To study the effect on CLL cells, we assessed the CD37 antibodies in parallel with Rituximab and Alemtuzumab in a whole blood culture system (n=21). In addition, the effect of the antibodies was assessed after in vitro treatment in cell culture by FACS (7-AAD+Annexin).
With increasing concentrations (0.01-100μg/ml) of mAb 37.1 and mAb 37.2 we found effective CLL cell depletion in the whole blood assay. As shown in figure 1 CLL cell depletion was observed with 1μg/ml mAb 37.1 and 37.2 at 3h and 8h. The comparison of mAb 37.1 and mAb 37.2 showed higher CLL cell depletion with mAb 37.1 (1μg/ml mAb 37.1 3h: 52.2% alive cells vs. mAb 37.2 3h: 71.5%). Maximum CLL cell depletion was observed at 10μg/ml (23.1% alive cells (mAb 37.1) vs. 46.7% alive cells (mAb 37.2)). Incubation with mAb 37.1 and mAb 37.2 consistently led to homotypic aggregation. Both CD37 Abs were significantly more effective in depleting CLL cells than Alemtuzumab (89.4% alive cells at 3h (10μg/ml)(p<0.001)) and Rituximab (94.8% alive cells 3h (10μg/ml)(p<0.001)).
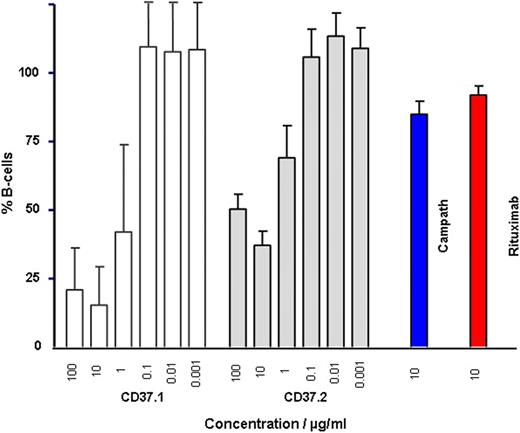
After 8h of incubation with mAb 37.1 and mAb 37.2 CLL cell depletion was highly efficacious with 15.4% and 37.1% remaining B-cells. This compared to moderate CLL depletion in this whole blood assay with Campath and Rituximab (85% and 92% remaining CLL cells respectively).
We were particularly interested in the effect in cases with TP53 mutation or deletion and fludarabine refractory cases. To this end we compared the CLL cell depletion in this high risk group (n=10) and the remaining patients (n=11). For both antibodies the CLL cell depletion was highly effective in low and high risk groups (i.e. 10μg/ml mAb 37.1 mean remaining CLL cells: 21.3% (8h) high risk; 11.1% low risk; 10μg/ml mAb 37.2 mean remaining CLL cells: 43.1% (8h) high risk; 33.2% low risk).
Both CD37 antibodies studied showed highly effective CLL cell depletion irrespective of genetic risk category. Our data suggest that CD37 antibodies could be more effective than CD20 or CD52 antibodies in CLL. CD37 Abs should be investigated in clinical trials.
CLL depletion with different Abs in whole blood assay after 8h. Concentrations in μg/ml
CLL depletion with different Abs in whole blood assay after 8h. Concentrations in μg/ml
Zenz: Boehringer: Honoraria. Heider: Boehringer Ingelheim: Employment. Reichardt: Boehringer Ingelheim: Employment. Stilgenbauer: Boehringer, Roche, Celgene, GSK,: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal