Abstract
Abstract 2364
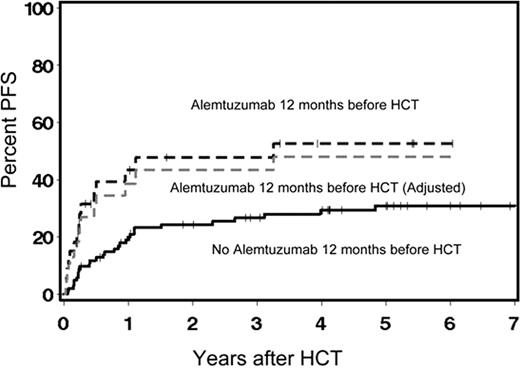
Allogeneic HCT using nonmyeloablative regimens may provide long-term remission in high-risk relapsed CLL. Here, we investigated the impact of histology, disease status, lymph node (LN) size, genomic aberrations, donor type, and prior alemtuzumab on outcomes. Pts (n=136) were conditioned with 2Gy TBI alone (12%) or 2Gy TBI plus 90 mg/m2 fludarabine (88%). Median age was 56 (range 42–72) years and median number of prior regimens was 4. Ninety percent of pts had fludarabine-refractory CLL. Incidences of grades II, III, and IV acute GVHD were 39%, 14%, and 2% respectively, and chronic extensive GVHD was 51%. Complete (CR) and partial remissions (PR) were seen in 55% and 15% of pts, respectively. Estimated 5-years rates of non-relapse mortality (NRM), progression/relapse, OS, and progression-free survival (PFS) were 32%, 36%, 41%, and 32%, respectively. Overall, 58 pts are alive; 45 in CR, 5 in PR, 5 with stable disease, and 3 with progression (PD)/relapse. Univariate outcomes (Table 1) were not statistically significantly different between donor types for NRM (p=0.37), relapse (p=0.17), or PFS (p=0.88). Pts with CLL and SLL had comparable rates of relapse and PFS. Disease status at HCT had no impact on NRM (p=0.75), relapse (p=0.31), or PFS (p=0.27). Relapse (p=0.51) and PFS (p=0.45) were not statistically significantly different among the 6 groups of cytogenetic abnormalities (Table 1). Both alemtuzumab within 12 months before HCT (53% vs. 31%, p=0.007, Figure) and LN size ≥5 cm (59% vs. 28%, p=0.003) were associated with increased rates of relapse. In Cox regression model for outcomes, prior alemtuzumab (HR: 2.20, p=0.02) and LN size ≥5 cm (HR: 2.21, p=0.02) were independently associated with increased relapse; while donor type, cytogenetic abnormalities, and disease status were not. PFS was also worse for pts with prior alemtuzumab (HR: 1.55, p=0.09) and LN size ≥5 cm (HR: 1.64, p=0.06). In multivariate models, pts who had alemtuzumab within 3 months prior to HCT appeared to have the highest relapse risk (Table 2). Prior alemtuzumab had no impact on CD3 donor chimerism following HCT. Further studies are warranted to explore whether the negative impact of alemtuzumab on relapse was due to unrecognized high-risk disease features or hampering the quality of graft-versus-tumor effects such as by deletion of host dendritic cells (Blood. 2002; 99: 2586). Allogeneic nonmyeloablative HCT is associated with graft-versus-leukemia effects even against chemo-refractory and high cytogenetic-risk diseases. We currently are studying novel approaches to better debulk disease before HCT and/or to augment graft-versus-leukemia effects after HCT for CLL pts with large LN size.
Univariate outcomes in 136 patients with CLL receiving nonmyeloablative HCT
| . | . | . | 5 year outcomes (%) . | ||
|---|---|---|---|---|---|
| Factor . | Group . | N . | NRM . | Rel . | PFS . |
| Donor | HLA-matched related | 75 | 26 | 43 | 31 |
| HLA-matched unrelated | 53 | 42 | 24 | 34 | |
| HLA-antigen mismatched | 8 | 15 | 56 | 29 | |
| p-value | 0.37 | 0.17 | 0.88 | ||
| Disease status at HCT | Chemo-responsive | 55 | 31 | 32 | 38 |
| Chemo-refractory | 72 | 32 | 41 | 27 | |
| Untested relapse | 9 | 36 | 22 | 42 | |
| p-value | 0.75 | 0.31 | 0.27 | ||
| Cytogenetic abnormalities | Normal | 39 | 42 | 25 | 34 |
| Del17p | 24 | 49 | 32 | 18 | |
| Del11q | 19 | 31 | 40 | 29 | |
| Tri12 | 10 | 20 | 40 | 40 | |
| Del13q | 18 | 17 | 43 | 40 | |
| Other | 26 | 21 | 47 | 33 | |
| p-value | 0.25 | 0.51 | 0.45 | ||
| Alemtuzumab within 12 months before HCT | No | 103 | 34 | 31 | 35 |
| Yes | 33 | 22 | 53 | 25 | |
| p-value | 0.80 | 0.01 | 0.07 | ||
| LN size | <5 cm | 36 | 30 | 28 | 39 |
| ≥5 cm | 100 | 33 | 59 | 11 | |
| p-value | 0.95 | 0.003 | 0.01 | ||
| . | . | . | 5 year outcomes (%) . | ||
|---|---|---|---|---|---|
| Factor . | Group . | N . | NRM . | Rel . | PFS . |
| Donor | HLA-matched related | 75 | 26 | 43 | 31 |
| HLA-matched unrelated | 53 | 42 | 24 | 34 | |
| HLA-antigen mismatched | 8 | 15 | 56 | 29 | |
| p-value | 0.37 | 0.17 | 0.88 | ||
| Disease status at HCT | Chemo-responsive | 55 | 31 | 32 | 38 |
| Chemo-refractory | 72 | 32 | 41 | 27 | |
| Untested relapse | 9 | 36 | 22 | 42 | |
| p-value | 0.75 | 0.31 | 0.27 | ||
| Cytogenetic abnormalities | Normal | 39 | 42 | 25 | 34 |
| Del17p | 24 | 49 | 32 | 18 | |
| Del11q | 19 | 31 | 40 | 29 | |
| Tri12 | 10 | 20 | 40 | 40 | |
| Del13q | 18 | 17 | 43 | 40 | |
| Other | 26 | 21 | 47 | 33 | |
| p-value | 0.25 | 0.51 | 0.45 | ||
| Alemtuzumab within 12 months before HCT | No | 103 | 34 | 31 | 35 |
| Yes | 33 | 22 | 53 | 25 | |
| p-value | 0.80 | 0.01 | 0.07 | ||
| LN size | <5 cm | 36 | 30 | 28 | 39 |
| ≥5 cm | 100 | 33 | 59 | 11 | |
| p-value | 0.95 | 0.003 | 0.01 | ||
Note: p-values reflect underlying hazard ratios over all follow-up period.
Impact of prior alemtuzumab on relapse after allogeneic HCT
| Alemtuzumab prior to HCT . | Number of pts . | Relapse rate at 5-years, % . | Univariate HR . | p-value . | Multivariate HR* . | p-value . |
|---|---|---|---|---|---|---|
| No or beyond 12 months | 103 | 31 | 1.0 | 1.0 | ||
| Within 3 months | 11 | 58 | 2.49 | 0.04 | 3.36 | 0.02 |
| Within 3.1-6 months | 13 | 46 | 2.43 | 0.07 | 2.29 | 0.10 |
| Within 6.1-12 months | 9 | 49 | 2.2 | 0.14 | 1.32 | 0.65 |
| Alemtuzumab prior to HCT . | Number of pts . | Relapse rate at 5-years, % . | Univariate HR . | p-value . | Multivariate HR* . | p-value . |
|---|---|---|---|---|---|---|
| No or beyond 12 months | 103 | 31 | 1.0 | 1.0 | ||
| Within 3 months | 11 | 58 | 2.49 | 0.04 | 3.36 | 0.02 |
| Within 3.1-6 months | 13 | 46 | 2.43 | 0.07 | 2.29 | 0.10 |
| Within 6.1-12 months | 9 | 49 | 2.2 | 0.14 | 1.32 | 0.65 |
Adjusted for genomic features, lymph node size, disease status at HCT, and donor type.
Relapse rate of 53% vs. 31% (p=0.007) among 136 CLL pts who did or did not receive alemtuzumab within 12 months prior to nonmyeloablative HCT. Adjustment for pre-transplant risk factors did not change the significant difference in relapse rate between the two groups.
Relapse rate of 53% vs. 31% (p=0.007) among 136 CLL pts who did or did not receive alemtuzumab within 12 months prior to nonmyeloablative HCT. Adjustment for pre-transplant risk factors did not change the significant difference in relapse rate between the two groups.
Off Label Use: All discussions about therapeutics used for HCT preparative regimens are off-label.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal