Abstract
Abstract 2357
There is ample evidence that poor-risk CLL, as defined by fludarabine refractoriness or the presence of deletion 17p-, can be successfully treated by allogeneic stem cell transplantation (alloSCT). It is unknown, however, whether alloSCT can also overcome the treatment resistance associated with TP53 mutations seen under conventional fludarabine combination therapy. Therefore we have assessed the impact of TP53 mutations on the outcome of alloSCT with the patient cohort enrolled on the CLL3X trial of the German CLL Study Group.
The CLL3X trial included 90 patients with poor-risk CLL who were allografted with unmanipulated blood stem cells from related or unrelated donors after nonmyeloablative conditioning. With a median follow-up of 46 months, 4-year event-free (EFS) and overall survival (OS) was 42% and 65%, respectively (Blood July 1, 2010). PFS and OS of 13 patients with deletion 17p- were similar to that of patients without this abnormality. TP53 mutations were identified by denaturating high-performance liquid chromatography (DHLPC) (exons 4–10). In addition, cases with deletion 17p- where no TP53 mutation was detected were also directly sequenced.
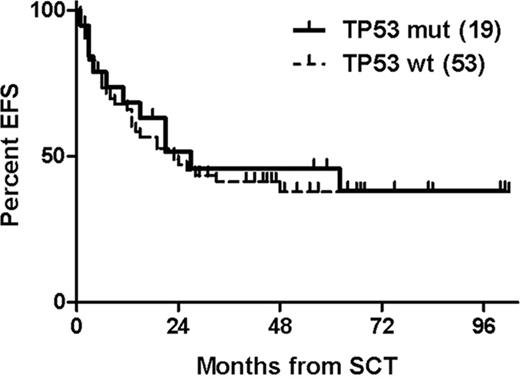
The TP53 mutational status could be obtained in 72 of 90 patients who had informative DNA samples from the time of study entry available. Of these, 19 (26%) showed TP53 mutations; 7 (10%) with a concurrent deletion 17p-, and 9 (12%) in the absence of deletion 17p-. 17p- status was not available in three TP53-mutated patients (4%). Three additional patients (4%) had 17p- without TP53 mutation. Four-year EFS and OS was 46% and 56% with TP53 mutation vs 38% and 66% without TP53 mutation (Figure). Within the TP53-mutated group, 4-year EFS and OS was 44% and 56% for patients without deletion 17p- vs 38% and 50% for patients with concurrent deletion 17p-. None of these differences were statistically significant. Among the patients who were event-free 12 months post alloSCT and had results of minimal residual disease (MRD) assessment available, the probability of being MRD-negative at this landmark was 71% with TP53 mutations and 63% without (p = 1.0). Finally, multivariate analysis using Cox regression modeling (adjusting for age, deletion 17p-, remission status at alloSCT, and T cell depletion) did not show a significant impact of TP53 mutations on EFS (Hazard ratio (HR) 0.71; 95%CI 0.31–1.61) and OS (HR 1.13; 95%CI 0.41–3.12).
AlloSCT can provide long-term EFS in about 40% of patients with poor-risk CLL with TP53 mutation independent from the presence of concurrent deletion 17p-. Disease control appears to be similar in patients with and without TP53 mutation, suggesting that alloSCT can overcome the treatment resistance associated with this abnormality.
Stilgenbauer: Amgen: Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Sanofi Aventis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal