Abstract
Abstract 2313
Immunologic reconstitution following stem cell transplantation (SCT) arises from thymic-independent peripheral expansion of memory T-cells transplanted with the stem cell graft, and thymic-dependent maturation of stem cell derived lymphoid progenitor cells. As a consequence of thymic involution, adult stem cell transplant recipients rely largely upon the thymic-independent pathway. This process is facilitated by a plentiful supply of mature T-cells contained within peripheral blood or bone marrow grafts. Umbilical cord blood (UCB) grafts contain predominantly naive T-cells thereby limiting the potential for thymic-independent immune recovery. Recent reports have documented slow immune recovery in adult UCB recipients, however it has yet to be described in the context of a comparable population of adult allogeneic matched sibling or unrelated donor SCT.
Between 2007 and 2009, we characterized cellular immune reconstitution in a consecutive cohort of adult patients undergoing myeloablative SCT using either matched sibling (MSD), matched unrelated donor (MUD) peripheral blood stem cell (PBSC) or dual UCB donor grafts. Dual UCB transplant recipients were conditioned with TBI 1350cGy and fludarabine 160mg/m2 (Flu). PBSC recipients were conditioned with either TBI 1350cGy or IV busulfan 12.8mg/kg (Bu) and cyclophosphamide 120mg/kg, or Bu/Flu. PBSC grafts were allele-level matched at HLA-A, B, C and DRB1. UCB grafts were mismatched at no more than 2 class I or class II loci. Both cohorts received GvHD prophylaxis with Tacrolimus for at least 6 months. UCB recipients received mycophenolate mofetil 2g/day for at least 60 days instead of standard dose methotrexate GvHD prophylaxis for PBSC recipients. Quantification of the following lymphoid subsets were performed by flow cytometry on fresh peripheral blood prior to, and then at approximately 45, 100, 180, and 360 days following transplantation: NK and NKT cells, B-cells, plasmacytoid dendritic cells, CD3+, CD4+, CD8+, regulatory and na•ve T-cells. T-cell receptor excision circles (sjTREC) were quantified by real time PCR on DNA collected from an isolated fraction of CD3+ T-cells. Cumulative corticosteroid usage prior to day 100 (as determined by area under the curve) was determined as a method for comparing acute GvHD severity. The Kruskal-Wallis test with adjustments for multiple comparisons was used compare immune recovery values at 45, 100, 180 and 360 days post transplantation. The PearsonÕs Chi-square test was used to compare the CMV reactivation rate of UCB to MSD/MUD.
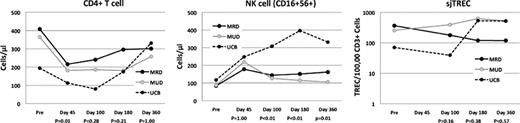
Forty-two recipients of allogeneic SCT (MSD-13, MUD-14, UCB-15) with successful donor engraftment and who survived a minimum of 6 months were evaluated. The MSD, MUD and UCB recipients had a median age of 41, 42 and 31 years, respectively (p = 0.13). There was no significant difference in corticosteroid administration before day 100 or chronic GvHD in the three cohorts (p=0.74). There were no differences in the kinetics of immune recovery between MSD and MUD recipients. At 45 days following transplantation, UCB recipients had significantly lower levels of CD4+ (figure), CD8+ and NKT-cells. By day 100, UCB recipients had similar numbers of CD4+ T-cells, but remained lower in the other major T-cell subsets. By day 180 however, all T-cell subsets except NKT cells (p=0.033) were similar to the MSD/MUD recipients. NK cell recovery was faster in the UCB recipients (figure). There was also no difference in B-cell recovery among the 3 cohorts. TREC levels were not significantly different in recipients of all graft types, but were uniformly low (figure). The slow recovery of T-cells in the UCB cohort corresponds with a higher Kaplan-Meier estimate of CMV reactivation compared to the MSD/MUD cohort (90% vs. 36%; p = 0.0078).
Compared to HLA-identical MSD and MUD adult SCT recipients, quantitative lymphoid recovery in UCB transplant recipients is slower in the first two months, but these differences are erased by 4–6 months following transplantation. NK reconstitution is more rapid in UCB recipients. Reduction of early opportunistic infections affecting UCB transplant recipients may arise from effective techniques to boost early T-cell recovery.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal