Abstract
Abstract 2312
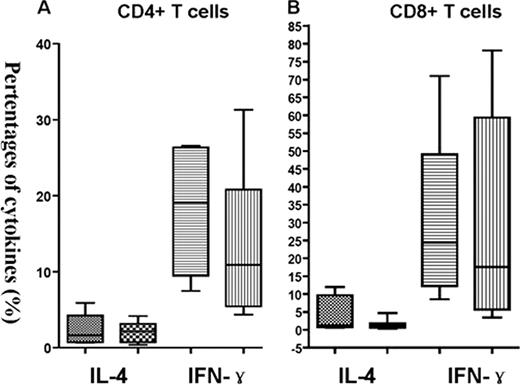
Unmanipulated human leukocyte antigen (HLA)-mismatched/haploidentical trasnplantation is an established treatment for patients without HLA-matched related or unrelated donors. In contrast to HLA-matched transplant, intensified immnological suppression, including antithymocyte globulin was used to overcome the HLA barrier. However, it is currently unclear how this radically different transplantation strategy affect immunological recovery. To investigate the immune reconstituion following unmanipulated human leukocyte antigen (HLA)-mismatched/haploidentical trasnplantation and HLA-matched transplantation. Seventy-five patients underwent transplantation from either HLA-identical siblings (25 cases) or haploidentical donors (50 cases) were enrolled in this prospective study. Recovery of T-, B-, monocytes, and dendritic cell subsets, proliferative of T lymphocytes in vitro response to mitogens, were investigated. Our results showed that in the first 90 days after grafting, counts of the following T cell subsets were signifcantly lower in haploidentical transplant recipients than those of HLA-matched transplant recipients: total CD4+ T cells, and their CD45RA positive (naïve), CD45RO (memory) subpopulation. After this interval, increases in CD4+, CD4+ naïve, and CD4+ memory T cell counts were observed in surviving subjects, by 1 year after transplantation, there were no differences in the numbers of recovered CD4+, CD4+ naïve, and CD4+ memory T cells between patients receiving haploidentical transplant and those receiving HLA-identical transplantation. In contrast, total counts of CD8+ T cells declined after conditioning and were significantly reduced by day 30 post-haploidentical transplantation. Thereafter, absolute of CD8+ T cell numbers expanded dramatically, and were signifciantly higher than that of HLA-identical recipients since day 90 post transplantation time point (Figure). CD3+ cells, CD8+ naïve, and CD8+ memory T cells were comparable by 90 days after transplantation, although lower numbers of these cells were found in haploidentical group prior day 90 after grafting. Furthermore, the ratio of CD4/CD8 T cells was significantly inverted in both groups untill 1 year after transplantation. While monocytes recovered promptly and reached normal levels by day 15 after haploidentical transplantation, though they also declined slightly by the 1 year time point, at which CD4+ T cell counts rebounded. These results indciate that quantitative CD4+ T-cell recovery is delayed after haploidentical transplantation, they also suggest that compensatory expansion of cytotoxic T lymphocytes and monocytes may accompany CD4+ T lymphopenia. Subsets of DC, including myeloid DC 1 (MDC1), MDC2 and plasmacytoid DC (pDC), in haploidentical recipients on day 15, and day 30 post allografting were significantly lower than those in HLA-matched recipients. No sigificant difference in the counts of B cells at any time point after transplantation in haploidentical recipients and HLA-matched recipients were found. On day 15 after transplantation, the expression of CD28 on CD8+ T cells was sigificantly lower in patients receiving haploidentical transplantation, then increased promptly and signifcantly higher than those receiving HLA-matched transplant on day 30, and 90, after this two time point the expression of CD28 were comparable between two groups. Moreover, the expression of CD28 on CD4+ T cells was also signifcantly higher than those receiving HLA-matched transplant on day 30, and 90. While only at days 30 post transplant, the expession of CD80 on pDC were signifcantly higher in patients receiving haploidentical transplant and than those receiving HLA-identical transplantation. The ability of the patient-derived T cells to produce IFN-Ã and IL-4 by day 30 after transplantation was similar in in patients without aGVHD between haploidentical transplant recipients and HLA-matched recipients. Our results suggest that comparable immune reconstitution could be achieved folloing hapolidentical transplantation and HLA-matched transplantation, this is related to the similar transplant outcomes.
The capability of T cells to produce IFN-Ã and IL-4 in patients without aGVHD between HLA-matched transplantation (the former box) and haploidentical transplantation (the latter box).
The capability of T cells to produce IFN-Ã and IL-4 in patients without aGVHD between HLA-matched transplantation (the former box) and haploidentical transplantation (the latter box).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal