Abstract
Abstract 2246
Plerixafor is a recently FDA approved antagonist of the CXCR4 chemokine receptor, which dramatically increases peripheral blood (PB) CD34+ cell counts during G-CSF mobilization of lymphoma and myeloma patients (pts, DiPersio et al, 2009 Blood). The pt subset that would optimally benefit from plerixafor administration has not been well characterized. We evaluated the cost-effectiveness of a “just-in-time” strategy of plerixafor administration to pts with poor mobilization of CD34+ cells during G-CSF administration.
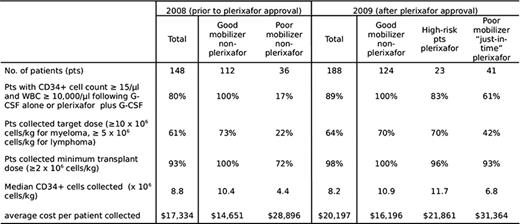
Charts of 148 consecutive lymphoma and myeloma pts in whom mobilization was attempted prior to the 2009 FDA-approval of plerxiafor were reviewed and compared to the charts of 188 pts mobilized in 2009. Prior to the availability of plerixafor, 36 pts (24%) were deemed poor mobilizers, defined as pt with < 15 CD34+ cells/μl blood and white blood cell counts (WBC) > 10,000/μl following at least 5 days of ≥10 ug/kg G-CSF. 10 pts (7% of total) failed to collect a minimum transplant cell dose of 2 × 106 CD34+ cells/kg. Following FDA approval, plerixafor was given to 64 of 188 patients (36%) deemed to be at risk for mobilization failure: 41 poor mobilizers who received plerixafor during continued G-CSF administration (“just-in-time” pts), and 23 “high-risk” pts who received plerixafor and G-CSF due to prior mobilization failure with G-CSF alone (n=7) or poor marrow reserve due to extensive prior therapy (n=16). Apheresis yields and the total costs (including drugs, apheresis and cryo-preservation charges, and one-time charge for thawing and stem cell administration) were calculated for pts who began apheresis with collection of a minimum transplant dose of CD34+ cells.
Among the total of 64 plerixafor-treated “high-risk” and “just-in-time” pts blood CD34+ counts increased from a mean of 8 cells/μl to 50 cells/μl, and the proportion of pts who initiated apheresis with ≥ 15 CD34+ cells/μl was 83% in the “high-risk” group and 61% in the “just-in-time” group. A minimum CD34+ cell dose of ≥ 2 × 106 cells/kg was collected from 93% of the “just-in-time” pts and 41% collected a target cell dose of either 5 × 106 CD34+ cells/kg (lymphoma) or 10 × 106 CD34+ cells/kg (myeloma). The data using plerixafor compared favorably with poor-mobilizers collected with G-CSF alone in which only 22% collected the target cell dose and 28% failed to mobilize enough cells for transplant. Mobilization with G-CSF alone results in an average cost of $17,334 for stem cell collection and administration. The selective administration of plerixafor to 64 pts at risk for mobilization failure resulted in an average cost of $20,197 for stem cell collection and infusion in all 188 pts, with collection of more than the minimum transplant CD34+ cell dose in 98% of pts. The average cost for the subset of 64 plerixafor-treated pts was slightly lower ($27,949) than that for the 36 non-plerixafor-treated poor-mobilizers ($28,896). Among the pts who underwent autologous stem cell transplant there was no difference in median days to ANC or platelet engraftment between plerixafor-treated and non-treated pts (data not shown).
Demographics, mobilization characteristics and costs in lymphoma and myeloma patients undergoing auto-transplant
A strategy of selective administration of plerixafor to pts at high-risk for mobilization failure based on treatment history or low blood CD34+ cell counts (<15 cells/ul in the face of WBC > 10,000 cells/μl after at least 5 days of G-CSF administration) can effectively increase the overall success rate for stem cell mobilization and does not substantially increase overall costs associated with stem cell collection.
No relevant conflicts of interest to declare.

This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal