Abstract
Abstract 2036
Maintenance of a reducing redox balance is a critical physiologic function of red cell metabolic machinery. Perturbation of this balance, whether inherited or acquired, is found in a variety of red cell pathologies. Methods for evaluation of red cell redox status include direct approaches such as determining glutathione (GSH, GSSG) levels, and indirect approaches such as measuring fluorescence of oxidation sensitive dyes. Here we describe an alternative method for evaluation of red cell redox status that can be used in vivo and in real-time assays. Engineered variants of GFP possessing two solvent accessible cysteine residues function as molecular redox sensors with distinct fluorescence characteristics. Excitation spectrum shifts upon the oxidation of cysteine residues forming a disulfide. A higher ratio of fluorescence when comparing excitation at 405nm versus 488nm indicates rising levels of oxidized GFP and a shift in cellular redox status. To validate redox GFPs in erythroid cells, we first performed in vitro assays with MEL cells over-expressing several related GFP sensors (ro-GFPs), selecting the brightest molecule (roGFP2) for further study. The sensor function of roGFP2 in MEL cells was verified by stimulation with exogenous oxidant (1mM H202) or reductant (10 mM DTT) as shown in the figure below. In order to create a physiologic in vivo model for study of red cell redox status, transgenic mice expressing roGFP2 specifically in the erythroid lineage were generated. roGFP2 expressing red cells demonstrate the expected shift in fluorescence upon exposure to H202 or t-butyl peroxide in a short-term assay. In vivo, we have measured red cell lifespan (using biotin-labeling) in roGFP2 transgenic animals to follow redox status of red cells as a function of cell age. Expression of roGFP2 has no effect on red cell survival. Interestingly, when comparing old red cells (age > 50days) with younger cells (age < 50days), a shift in GFP fluorescence ratio indicating that a higher fraction of the sensor is oxidized in the aged cells was observed. This observation is consistent with the hypothesis that metabolic changes, in particular a decline in ability to reduce oxidative damage, contribute to red cell senescence. We are generating several murine strains with defined red cell defects also expressing roGFP2 in order to assess the role of changes in intra-erythrocyte redox status in a range of pathologic conditions. In vitro and in vivo assays using roGFP2 transgenic cells/mice are in process to determine the potential utility of this system as a screen for hematoxicity of drugs and other compounds.
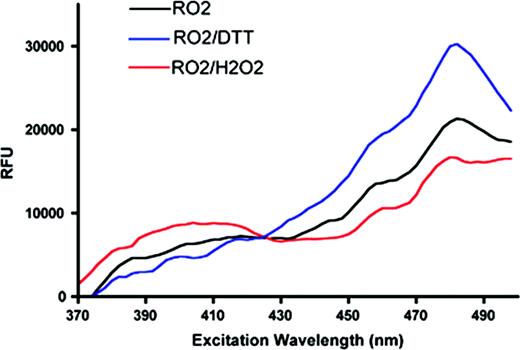
Evaluation of roGFP2 function in MEL cells. The Y-axis shows fluorescence emission as a function of excitation wavelength (X-axis)—showing a shift when cells are exposed to oxidizing or reducing conditions.
Evaluation of roGFP2 function in MEL cells. The Y-axis shows fluorescence emission as a function of excitation wavelength (X-axis)—showing a shift when cells are exposed to oxidizing or reducing conditions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal