Abstract
Abstract 2
Heme, a complex of iron and protoporphyrin IX, is ubiquitous in aerobic cells, serving as the prosthetic group for essential hemoproteins. However, an excess of intracellular “free” heme is toxic to cells, promoting lipid peroxidation, membrane injury, reactive oxygen species (ROS) production, and ultimately apoptosis, necessitating tight regulation. FLVCR is a human erythroid progenitor cell membrane transporter that exports heme from the cytosol, thereby protecting cells from heme toxicity (Quigley et al. Cell, 2004). It is also highly expressed at sites of heme trafficking including the liver, gut, kidney and erythrophagocytic macrophages. In accordance with its function and expression pattern, our knockout mouse model demonstrates that FLVCR is important both for erythropoiesis and for systemic iron homeostasis (Keel et al. Science 2008). Female mosquitoes ingest ∼10 times their weight in blood in a single meal, thus large quantities of heme are released into the midgut lumen, a source of toxic ROS. Notably, hematophagy also represents a major bottleneck in the Plasmodium lifecycle. Gametes egressing from disintegrating RBCs are fertilized within the midgut lumen, forming small numbers of ookinetes that are susceptible to ROS as they traverse the mosquito midgut epithelial cells (to develop into oocysts). Indeed, numerous studies demonstrate that mosquitoes with increased midgut oxidative stress are resistant to Plasmodium transmission. We hypothesize that dysregulation of anopheline FLVCR–mediated heme export by the mosquito midgut epithelium will increase epithelial cell oxidative stress and impact Plasmodium transmission at its weakest point.
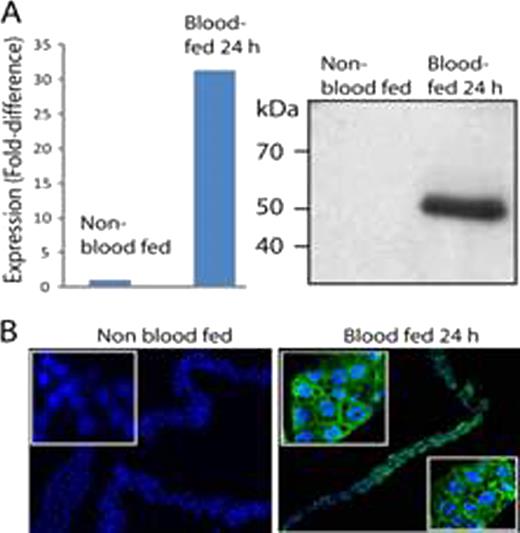
Using genomic approaches, we isolated anopheline orthologs of FLVCR from the prevalent malaria transmission vectors, Anopheles gambiae and A. stephensi. The AgFLVCR cDNA predicts a 51kDa protein of 474 aa; the sequence of AsFLVCR (474 aa) is 97% identical. Like FLVCR, both genes are predicted to encode Major Facilitator Superfamily transporter proteins containing 12 TM domains, with the N– and C–termini in the cytosol. Overexpression of AgFLVCR cDNA in (rat) NRK cells results in an increase in export of ZnMP (a fluorescent heme analog) comparable to that observed with NRK cells overexpressing human FLVCR—a rate significantly greater (∼2–fold) than that of control NRK cells, as measured by quantitative microscopy (n=4, p<0.001). Similar increases in heme export relative to controls are observed when (insect) Sf9 cells are transduced with Asflvcr or Agflvcr and ZnMP export analyzed using flow cytometry. The increase is blocked by specific siRNA against Asflvcr or Agflvcr. As expected, incubation with exogenous heme (20 μ M) reduces viability of NRK cells. However, overexpression of AsFLVCR in NRK cells, by reducing intracellular ROS levels by ∼ 40%, improves their viability 3–fold (n=3, p<0.001). In studies of A. stephensi mosquitoes, we demonstrate robust upregulation of AsFLVCR mRNA (Fig. A, left) and protein (right) at 24 h after hematophagy, while confocal fluorescence immunolocalization studies with an antibody (Ab) generated against an intracellular epitope of AsFLVCR show cell membrane targeting in midgut epithelial cells following a blood meal (Fig. B; blue, DAPI; green, AsFLVCR). Importantly, dsRNA-mediated knockdown of AsFLVCR in mosquitoes decreases midgut Asflvcr mRNA by 85%, resulting in a 30% reduction in protein levels post-hematophagy. A polyclonal Ab (α-AsFLVCR) was also generated against an extracellular epitope. Preliminary studies indicate it impairs heme export from Sf9 cells overexpressing AsFLVCR. At present we are evaluating the effects of α-AsFLVCR– or dsRNA–mediated blockade of AsFLVCR heme export function on Plasmodium oocyst survival, salivary gland sporozoite development, mosquito midgut ROS production and mosquito fecundity. If these results validate our hypothesis, modulation of anopheline FLVCR–like proteins may serve as a means of controlling malaria.
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal