Abstract
Abstract 1679
Aberrant expression of the wild-type transcript of interferon regulatory factor 8 (IRF8-WT) in hematopoietic stem cells is associated with differentiation block, inhibition of apoptosis, and leukemogenesis. We recently identified 3 novel transcript variants of IRF8 (IRF8-SVs). Each of the splice variants introduces a part of the terminal portion of intron 1 into the transcript, while splicing out exon 1 of the reference sequence (data submitted in a separate ASH abstract). Although these splice variants can be found at very low levels in hematopoietic cells from “healthy” adults, we have found that a subpopulation of AML patients expresses IRF8-SVs at much higher levels (>2-fold) than hematopoietic cells from normal donors. Therefore, we performed a retrospective study to examine the prognostic significance of IRF8-SV transcripts in previously untreated adult AML patients.
RNA from pretreatment bone marrow (BM) or peripheral blood (PB) was available in the Southwest Oncology Group (SWOG) repository for 194 patients with previously untreated AML. These patients received remission induction therapy with cytarabine and daunorubicin on SWOG trials S9031, S9126, S9333, or S9500. Compared to the other patients on these trials, the 194 patients had higher WBC counts (median 32,100/mcL vs. 7,300/mcL), BM and PB blast percentages (medians 70% vs. 60% and 44% vs. 20%, respectively) and were younger (median 61 vs. 66 years), more likely to have normal karyotype (44% vs. 34%), and less likely to have deletions of chromosomes 5q and/or 7q (11% vs. 25%). After adjusting for these factors, the 194 patients did not differ significantly in complete response (CR) or resistant disease (RD) rates, relapse-free survival (RFS), or overall survival (OS) from other trial participants. Quantitative RT/PCR (Q-RT/PCR) assays were developed to specifically quantify the collective expression of the three IRF8-SV transcripts. B-glucoronidase (GUSB) was used as endogenous control to correct for RNA integrity. Fold change was computed relative to the pool of RNA from the peripheral blood of 10 healthy donors using the 2-ΔΔCt method. All Q-RT/PCR assays were performed in duplicate with appropriate negative and positive controls. Geometric means of the duplicate fold-change values were used for all statistical analyses.
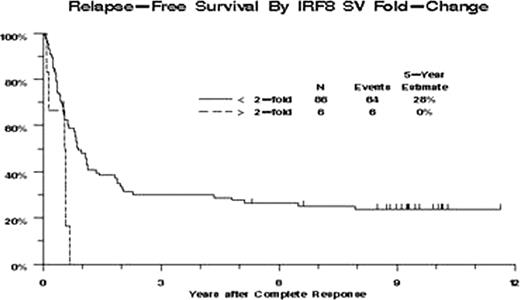
Increased IRF8-SVs expression (defined by >2-fold change) was present in 26 patients (13%) and was significantly associated with higher CD34 expression (P<0.0001), but not with WBC, blast percentages (BM or PB), normal karyotype, or FLT3 ITD. Patients with increased IRF8-SVs expression had lower CR rate (23% vs. 52%, P=0.010), higher RD rate (54% vs. 31%, P=0.044), reduced OS (hazard ratio [HR] = 1.41, P=0.14) and poorer RFS (HR=3.01, P=0.029). After adjusting for effects of age and CD34 expression, increased IRF8-SVs expression was significantly associated with poorer RFS (HR=2.94, P=0.015, Figure 1), but not with CR (odds ratio [OR] = 0.54, P=0.26), RD (OR=1.58, P=0.34) or OS (HR=1.00, P=1.00).
This is the first study examining the clinical importance of these novel IRF8 splice variants in malignant cells. We found that increased expression of IRF8-SVs was associated with a low CR rate and significantly inferior RFS in adult AML patients, suggesting that IRF8-SVs may be a novel and important AML prognostic biomarker. Additional studies are planned to examine the clinical and functional significance of the three novel IRF8 splice variants, individually and collectively, in a larger AML population and other hematopoietic malignancies.
Relapse-Free Survival. Kaplan-Meier estimates of RFS for 92 patients who achieved CR. Tick marks indicate censored observations.
Relapse-Free Survival. Kaplan-Meier estimates of RFS for 92 patients who achieved CR. Tick marks indicate censored observations.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal