Abstract
The importance of bone marrow aspiration and biopsy in the evaluation of hematopoietic and non-hematopoietic disorders is well established. However, this technique is associated with morbidity and mortality risks.1 Recently, a battery-powered bone marrow biopsy system was developed to allow operators to safely, quickly and efficiently access the marrow space. We previously evaluated this device in swine models and in patients needing routine hematology outpatient evaluation.2 In the current study we compared the powered device to the traditional manual technique by relatively assessing pain scores, procedure times, biopsy capture rates, quality of material retrieved, safety and operator satisfaction.
Two large academic medical centers participated in this trial (San Antonio, TX and Madrid, Spain). The study protocol was approved by each center's institutional review board. Adult patients requiring bone marrow biopsies were considered for the study. Following informed consent, patients were randomized to have procedures using a manual biopsy device (T-handle Jamshidi bone marrow biopsy and aspiration set, Cardinal Health, Dublin, OH) or the Powered device (OnControl 11 gauge/102mm Bone Marrow Biopsy System, Vidacare Corporation, Shavano Park, TX). After infiltration of the skin and medullary bone with local anesthesia, a visual analog scale (VAS) pain score was recorded immediately following skin puncture and once again at the end of the procedure for each patient. Procedure time was measured from skin puncture to core specimen ejection from the needle. Pathologic assessment of 30 randomized samples was carried out. Operator satisfaction with devices was measured on a scale of 0–10, with 10 as the highest rating. Statistics were calculated using t-test and chi-square, with an alpha-level of 0.05.
Five operators from 2 sites enrolled 50 patients (Powered, n=25; Manual, n=25). Of those patients, 58% were male and 42% were female; and had a mean age of 56.0±18.0 years. The mean height was 167.5 ± 10.5cm and the mean weight was 78.7 ± 22.7kg. Forty percent were lymphoma patients—the largest diagnostic group. Between patient groups, there were no significant differences in the means for these variables. See Table below for quantitative results, including pathology analysis. For the pathology qualitative analysis, there was no difference between groups for hemorrhage, clot/particle spicules, or smear spicules.
| . | Powered . | Manual . | p-value . |
|---|---|---|---|
| Mean VAS score, needle insertion | 2.6 ± 2.0 | 4.1 ± 2.5 | 0.022 |
| Mean VAS score, overall procedure | 3.2 ± 2.2 | 3.8 ± 3.0 | 0.438 |
| Mean biopsy procedure time (seconds) | 100.0 ± 72.8 | 224.1 ± 79.0 | <0.001 |
| First pass biopsy core capture success | 88% | 88% | 1.000 |
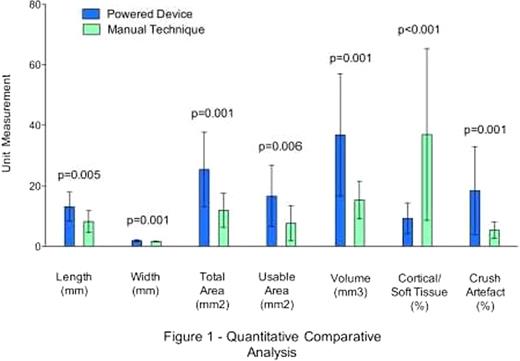
| Mean core biopsy length (mm) | 13.1 ± 4.8 | 8.2 ± 3.6 | 0.005 |
| Mean core biopsy width (mm) | 1.9 ± 0.3 | 1.6 ± 0.15 | 0.001 |
| Mean core biopsy usable area (mm2) | 25.4 ± 12.3 | 11.9 ± 5.6 | 0.001 |
| Mean core biopsy volume (mm3) | 36.8 ± 20.2 | 15.3 ± 6.2 | 0.001 |
| Mean core biopsy cohesiveness score | 2.1 ± 1.3 | 1.9 ± 0.7 | 0.597 |
| Mean core biopsy, percent cortex material | 5.1 ± 9.2 | 36.9 ± 28.3 | <0.001 |
| Mean core biopsy, percent crush artifact | 18.4 ± 14.5 | 2.7 ± 5.3 | 0.001 |
| Mean operator satisfaction score | 9.5 ± 0.5 | 7.0 ± 1.6 | <0.001 |
| . | Powered . | Manual . | p-value . |
|---|---|---|---|
| Mean VAS score, needle insertion | 2.6 ± 2.0 | 4.1 ± 2.5 | 0.022 |
| Mean VAS score, overall procedure | 3.2 ± 2.2 | 3.8 ± 3.0 | 0.438 |
| Mean biopsy procedure time (seconds) | 100.0 ± 72.8 | 224.1 ± 79.0 | <0.001 |
| First pass biopsy core capture success | 88% | 88% | 1.000 |
| Mean core biopsy length (mm) | 13.1 ± 4.8 | 8.2 ± 3.6 | 0.005 |
| Mean core biopsy width (mm) | 1.9 ± 0.3 | 1.6 ± 0.15 | 0.001 |
| Mean core biopsy usable area (mm2) | 25.4 ± 12.3 | 11.9 ± 5.6 | 0.001 |
| Mean core biopsy volume (mm3) | 36.8 ± 20.2 | 15.3 ± 6.2 | 0.001 |
| Mean core biopsy cohesiveness score | 2.1 ± 1.3 | 1.9 ± 0.7 | 0.597 |
| Mean core biopsy, percent cortex material | 5.1 ± 9.2 | 36.9 ± 28.3 | <0.001 |
| Mean core biopsy, percent crush artifact | 18.4 ± 14.5 | 2.7 ± 5.3 | 0.001 |
| Mean operator satisfaction score | 9.5 ± 0.5 | 7.0 ± 1.6 | <0.001 |
Results of this trial suggest that the use of a Powered bone marrow biopsy device significantly reduces needle insertion pain. While not reflected in the results, overall pain may be better tolerated due to the important difference in procedure time. Moreover, the superior size and overall quality of core specimens retrieved by the Powered device provides more material for pathologic evaluation, thereby increasing diagnostic yield and reducing the need for repeat procedures. Cohesiveness of the medullary bone sampled was comparable for both techniques; however, the Powered system was less likely to recover non-hematopoietic tissue (e.g. cortical bone and soft tissue). Artifact was slightly more common with the Powered device (aspiration, hemorrhage and crush) but this did not impact on the diagnostic quality of the sample. No differences in safety data were noted for either technique and operator satisfaction favored the Powered device.
1. Bain BJ. Bone marrow biopsy morbidity and mortality. British Journal of Haematology 2003;121:949-51.
2. Swords RT, Kelly KR, Cohen SC et al. Rotary powered device for bone marrow aspiration and biopsy yields excellent specimens quickly and efficiently. J Clin Pathol 2010;63:562-5.
Swords:Vidacare Corporation: Research Funding. Anguita:Vidacare Corporation: Research Funding. Kelly:Vidacare Corporation: Research Funding. Philbeck:Vidacare Corporation: Employment. Miller:Vidacare Corporation: Employment, Equity Ownership. Brenner:Vidacare Corporation: Research Funding.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal