Abstract
Abstract 1469
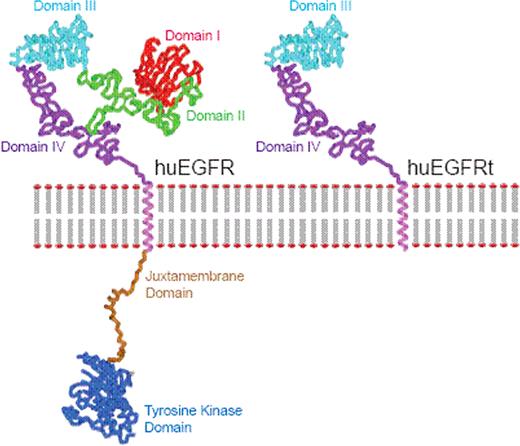
Hematopoietic cell-based therapies, including genetically manipulated cell products derived from either hematopoietic stem cells or T cells, is an emerging area in applied biotechnology. In both of these venues, a variety of genetic engineering approaches are being studied to endow cells with novel attributes, to increase their therapeutic potency and/or safety. Common to the field of ex vivo cellular genetic engineering is the need to purify cells that express desired quantities of therapeutic transgene(s) and cull out non-expressing cells that either lack transgene endowed therapeutic activity or safety features. However, current drug selection strategies are associated with prolonged ex vivo culture that drives terminal differentiation of the T cells, which has in turn been found to be associated with impaired antitumor efficacy of adoptively transferred CD8+ T cells in vivo. Thus, we were interested in developing a single transgene encoded polypeptide that can serve both as an ex vivo selection epitope and in vivo tracking marker/target for mAb-mediated cell ablation, while fulfilling the criteria of being functionally inert, non immunogenic, and amenable to commercially available cGMP-grade selection systems appropriate for clinical use. Here we describe a truncated human EGFR polypeptide (huEGFRt) devoid of extracellular N-terminal ligand binding domains and intracellular receptor tyrosine kinase domains. Retained features of huEGFRt include type I transmembrane cell surface localization and a conformationally intact binding epitope for pharmaceutical-grade anti-EGFR mAb, cetuximab/Erbitux™.
Applying this system to cellular immunotherapy, we designed lentiviral vector prototypes housing multifunctional constructs combining huEGFRt with CD19-specific chimeric antigen receptors (CARs), and demonstrate that biotinylated-cetuximab immunomagnetic selection of transduced human T cells results in coordinate enrichment of CAR+ cells from 2% to over 90%. The huEGFRt-mediated selection did not affect the phenotype (i.e., TCR, CD3, CD4, CD8, CD28, and granzyme A expression), the in vitro expansion potential, nor the in vivo engraftment fitness (upon transfer into immunodeficient mice) of the T cells. Direct examination of EGF-binding and phospho-tyrosine analysis confirmed that this selection marker is functionally inert and has no negative effect on the T cell product. In addition, cytotoxicity against B cell malignancies and IFN-g/TNF-a production through the CD19-specific CAR was dramatically enhanced in the huEGFRt-selected population. The utility of huEGFRt in tracking the gene modified, transferred cells in vivo within easily obtained human tissues such as blood, bone marrow and tissue biopsies was then also proven via detection of huEGFRt using multiparameter flow cytometric analysis or FDA approved immunohistochemical techniques/reagents. In addition, we were able to demonstrate that Erbitux™ could mediate ADCC of huEGFRt+ T cells in vitro and inhibit the growth of huEGFRt+ CTLL2 cells in NOD/Scid mice, supporting the use of huEGFRt as a suicide gene via cetuximab-mediated ADCC after adoptive transfer. Together these data suggest that huEGFRt is a superior selection marker for any transduction system that can be applied to the generation of cell products for hematopoietic cell-based medical therapies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal