Abstract
Abstract 1345
Multiple Myeloma (MM) plasma cells are known to posses low replicative potential and plasma cell labelling index is generally accepted as a strong prognostic factor. TC classification of MM defines categories of myeloma cases expressing different cyclins (Fonseca et all, Leukemia 2009). Although cyclin dependent kinase inhibitors (CDKI) are important for cell cycle, they are not included in this molecular classification.
With an aim to define high and low proliferative myeloma cases antibodies detecting cyclins (Cyc) A, D1, D2, D3, phosphorylated retinoblastoma (Rb), p16, p21, p27, Ki67 were applied to tissue sections obtained from either marrow plasmacytoma of 106 consequtive myeloma patients (median: 59, 32–79 years) diagnosed between 1998–2007. 100 patients <65 years (n:51) were evaluable for treatment outcome and received an induction of VAD (4-6 cycles), followed by a novel agent containing regimen (2-4 cycles) or autologous stem cell transplant (ASCT) depending on the response to induction. Postransplant consolidation or subsequent transplants were performed when < VGPR was obtained. Elderly patients (n:49) received induction for at least a year. Novel agents (Thalidomide: 66.7%, Bortezomib: 27.5%) were given in 94.2% of patients as ≥2 line treatment. Mann Whitney U, Kaplan-Meier or log rank analysis were performed using the SPSS version 15.0.
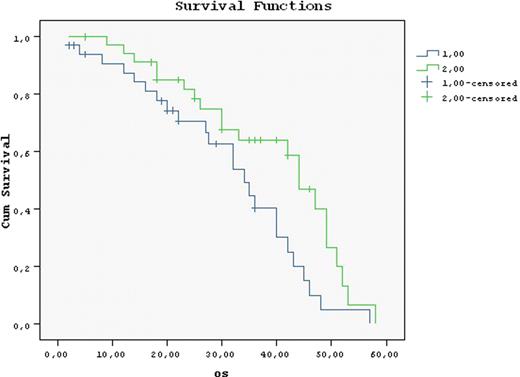
Loss of CDKI's were detected in 55.4–88.5 % of the cases. Cyclin D1D2D3 or Cyclin A expression was detected in 29.2, 21.7, 5.7 or 19.8 % of the cases. Rb was detected in 25%. Among the CDK's or CDKI's only Cyc D2 was correlated with Ki67 percentage (p=0.027). Loss of all CDKI's was observed in 31.1 %. The majority of p16 (-) cases were Cyc A (-) too (p=0.001). Loss of p16 and p 27 was observed more frequently than loss of p 21 (88 and 85% versus 54%). Patients were either both CycA and p21 (-) (50%) or both CycA and p21 (+) (31 %) (p=0.025). However there was no patient expressing Rb and p16. On the contrary, the majority patients were both Rb and p16 (-) (60%) (p=0.037). Groups of patients with high proliferative protential group 1 (Cyc D+ p16-), group 2 (CycA+ p21-), group 3 (CycA+ Rb+) and low proliferative protential group 4 (Rb- p16+), group 5 (CycA- Rb-) were analyzed. These were observed 42.5 %, 5.5%, 11.7%, 15.4% and 57.1%. No correlation could be found between the CDKI/Cyc defined risk groups and ISS, B2MG, LDH, CRP, OS. However among p16 (-) cases Rb (-) ones (n:39) had longer OS than Rb (+) patients (n:18) (49 vs 39 months). All p16 (+) patients were Rb (-) too and had a shorter OS (42 vs 84 months, p=0.006). Response to initial treatment was ≥ VGPR 16.8 %, ≥ PR 52%, refractory 21.4 %. High proliferative group 1 or patients with high LDH values had higher initial response rates (82.1 vs 53.8 %, p: 0.016 and 67.6 % vs 36 % p= 0.018). The deepest response was observed with initial treatment among 47.3 % and was improved following ASCT (18.3%). ASCT also improved OS among all (58 vs 34 months, p=0.0) but more strongly among Rb and/or CycA (-) patients (Table). The response to initial treatment did not influence OS. However if response is not upgraded following subsequent treatments OS deteriorated (5 year OS: 34 vs 44 months p=0.022) (Figure). These suboptimal responding patients could not be predicted by ISS, age, B2MG, high/low proliferating group definition. Although LDH was high in 72.7 vs 54.5 % of poor-responders this comparison was not significant.
While depth of response to therapy did not, improvement of response with subsequent therapy ie novel agents or ASCT prolongged OS (34 vs 44 months, p=0.022) and was associated with lower LDH values. Compared to gene expression profilling, tissue array using monoclonal antibodies is a method which can be more widely applicable and in our study was able to define new prognostic parameters. Overall ASCT extended OS. This effect was stronger among patients lacking Rb and/or Cyc A, This finding echoes the need for better treatment modalities for patients with poorer prognosis ie Rb and/or Cyc A positivity. Multivariate analysis results will be presented during the congress.
Presence of cell cycle related markers and overall survival (months)
| . | n . | months . | |
|---|---|---|---|
| ASCT (-) | Cyc A (+) | 8 | 31,6 |
| Cyc A (-) | 41 | 71 | |
| Rb (+) | 16 | 58,2 | |
| Rb (-) | 32 | 55,6 | |
| Cyc A (+) Rb (+) | 3 | 29 | |
| Cyc A (-) Rb (-) | 23 | 30,1 | |
| ASCT (+) | Cyc A (+) | 12 | 61,9 |
| Cyc A (-) | 39 | 91,3 | |
| Rb (+) | 11 | 54,4 | |
| Rb (-) | 40 | 91,5 | |
| Cyc A (+) Rb (+) | 6 | 46 | |
| Cyc A (-) Rb (-) | 34 | 56,9 | |
| . | n . | months . | |
|---|---|---|---|
| ASCT (-) | Cyc A (+) | 8 | 31,6 |
| Cyc A (-) | 41 | 71 | |
| Rb (+) | 16 | 58,2 | |
| Rb (-) | 32 | 55,6 | |
| Cyc A (+) Rb (+) | 3 | 29 | |
| Cyc A (-) Rb (-) | 23 | 30,1 | |
| ASCT (+) | Cyc A (+) | 12 | 61,9 |
| Cyc A (-) | 39 | 91,3 | |
| Rb (+) | 11 | 54,4 | |
| Rb (-) | 40 | 91,5 | |
| Cyc A (+) Rb (+) | 6 | 46 | |
| Cyc A (-) Rb (-) | 34 | 56,9 | |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal