Abstract
Abstract 1330
Veno-occlusive disease (VOD) remains a major cause of morbidity and mortality in children undergoing haematopoietic stem cell transplantation (HSCT) for thalassaemia. We investigated the impact of demographic characteristics, iron load (pre-transplant ferritin, hepatic iron concentration), hepatic fibrosis (Ishak staging), Pesaro risk class and defibrotide prophylaxis on the occurrence of VOD in 52 consecutive children (median age 6 years, range 2 – 18 years) undergoing myeloablative HLA-matched related donor HSCT for beta thalassaemia major (Pesaro class I n=27, class II n=23 and class III n=2). Following hypertransfusion to suppress endogenous haematopoiesis patients were conditioned with oral busulfan 14 mg/kg (days -9 to -6), cyclophosphamide 200 mg/kg (days -5 to -2) and alemtuzumab 0.3 mg/kg (days -8 to -6), except 2 Pesaro class III patients who received fludarabine instead of alemtuzumab. Graft versus host disease prophylaxis was ciclosporin for six months and methotrexate 10 mg/m2 on days +4 and +7. Forty patients had a liver biopsy prior to HSCT for Ishak staging of hepatic fibrosis.
VOD was diagnosed in 18 (35%) according to Seattle criteria. The median onset of VOD was day +10 post-HSCT (range: 6 – 27 days); one patient developed late VOD on day +27. All patients had weight gain and hepatomegaly and the median peak bilirubin was 39 μmol/L (17 - 196). 13 patients (72%) had USS evidence of ascites and 3 (16%) patients had pleural effusion. Only 2 patients had reversal of portal blood flow on Doppler study. All patients who developed VOD were treated with defibrotide. Three (17%) patients required admission to intensive care for strict fluid balance and diuretic infusion and/or respiratory support without mortality.
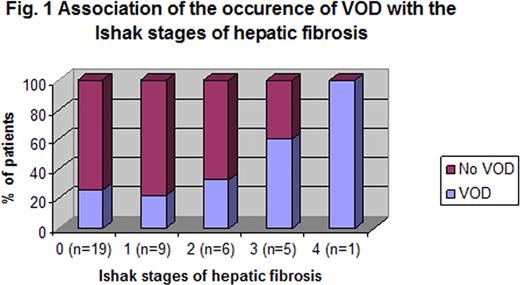
The incidence of VOD was highest in the pre-school children (10/22; 45%) compared to those aged 5–8 years (4/15; 27%) and > 8 years (4/15; 27%), although this difference was not statistically significant (p=0.13). No significant difference was noted in the gender (male=10/22; female=8/30, p=0.23) or ethnic origin (Middle-eastern=11/29; Asian=6/22, p=0.55). Pesaro risk class was associated with the occurrence of VOD: class I, 6/27 (22%); class II, 11/23 (47%), class III 1/2 (p=0.051). VOD occurred in 5/12 (41%), 7/20 (35%) and 2/7 (28%) of patients with hepatic iron <3 mg/g DW, 3–7 mg/g DW and >7 mg/g DW respectively (p=NS). The severity of hepatic fibrosis was significantly associated with the development of VOD: no fibrosis vs ≥ Ishak stage 3 fibrosis, p=0.024 (Fig. 1); stage 0 VOD 5/19 (26%); stage 1 VOD 2/9 (22%); stage 2 VOD 2/6 (33%); stage 3 VOD 3/5 (60%); and stage 4 VOD 1/1 (100%).
To reduce the risk of VOD risk-adjusted defibrotide prophylaxis was introduced (hepatic iron concentration >4 mg/g dry weight and/or hepatic fibrosis ≥ stage 2). 5/19 (26%) developed VOD before introduction of defibrotide prophylaxis compared to 13/33 patients (39%) developed VOD after the introduction of defibrotide prophylaxis (p=0.34). Of the patients who developed VOD, 2/5 (40%) patients developed multiorgan failure requiring intensive care admission pre introduction of defibrotide prophylaxis whereas only 1/13 (8%) did subsequently.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal