Abstract
Abstract 1276
Reactivation of cytomegalovirus (CMV) post allogeneic stem cell transplant (SCT) has shown to be controlled by the adoptive transfer of donor derived CMV-reactive T cells (CMV-T). A current method for the generation of CMV-T cells is CMV antigenic stimulation of donor peripheral blood mononuclear cells (PBMC), followed by isolation of interferon-gamma (IFN-g) secreting cells, by magnetic enrichment. The feasibility of generating a CMV-T cell product for clinical use, using PBMC from a G-CSF mobilised donor stem cell apheresis has yet to be fully determined.
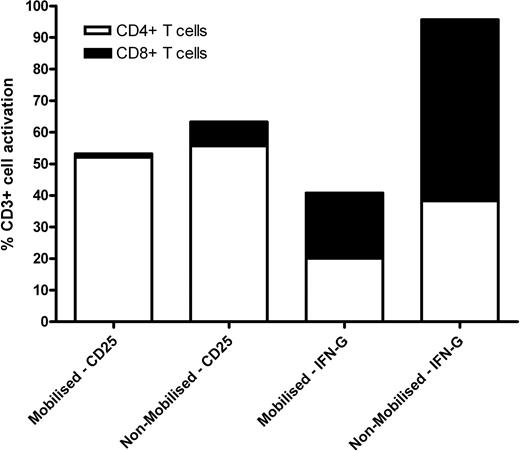
The proportion of CMVpp65 stimulated CD25+ T cells was equivalent between mobilised (53.15%) and non-mobilised (63.28%) PBMC. The CMV-T response was also predominantly CD4+ in both mobilised (98%) and non-mobilised (88%) PBMC. In contrast IFN-g secretion was shown to identify CMV-T in mobilised PBMC (41.77%), but this was consistently 50–60% lower in frequency compared to non-mobilised PBMC (95.59%). This 1–2 fold reduction in IFN-g+ T cell activation does not appear to effect the ratio of CD4+ to CD8+ T cells, which was comparable between mobilised (1:1.1) and non-mobilised (1:1.5) PBMC (Figure 1).
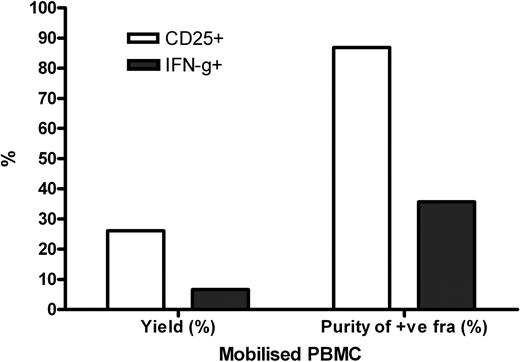
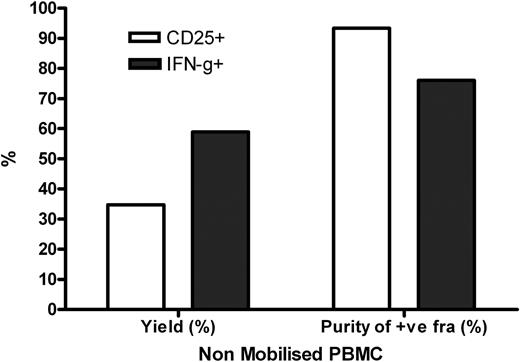
Enrichment of CD25+ CMV-T cells showed parity between mobilised and non-mobilised PBMC in terms of both yield (26.12% vs. 34.75%) and purity (89.92% vs. 93.39%). We have also observed that enrichment of CD25+ CMV-T cells depleted the naturally occurring CD25+ T cells seen in the unstimulated PBMC. Background expression of CD25 has been thought previously to be a barrier to selection of activated cells by CD25. However, CD25+ selections from non-stimulated cultures resulted in a yield of <1%.
These results indicate that G-CSF can suppress the Ag-specific T cell responses following in vitro stimulation with a CMVpp65 peptide pool but CMV-T can still be detected and isolated. We hypothesise that the reduced IFN-g+ T cell activation in mobilised PBMC could be as a result of the suppressive nature of an increased frequency of FoxP3+ IL-10 secreting T cells compared to that in non-mobilised PBMC.
We have demonstrated G-CSF suppression of CMVpp65 stimulated T cell responses with reference to a type 1 cytokine profile. Both CD25+ and IFN-g+ CMV-T from mobilised and non-mobilised PBMC are being tested for secretion of a profile of type 1 and type 2 cytokines to include TNF-α, IL-2, IL-4 and IL-10. Direct selection of CMV-T by CD25 expression represents a potential approach for isolating these cells for clinical use.
ES, KN and this work were supported by a grant from the Technology Strategy Board (TSB) of the UK Government Department of Business, Innovation & Skills
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal