Abstract
Patients with mantle cell lymphoma (MCL) typically respond to initial treatment but subsequently relapse. This pattern suggests that a population of MCL cells is both drug resistant and capable of clonogenic growth. The intracellular enzyme retinaldehyde dehydrogenase (ALDH) provides resistance to several toxic agents. ALDH can also identify stem cells in normal adult tissues and tumorigenic cancer stem cells in several human malignancies. We studied ALDH expression in MCL and found small populations of ALDH+ cells that were highly clonogenic. Moreover, ALDH+ MCL cells were relatively quiescent and resistant to a wide range of agents. Normal B cells can be activated by specific unmethylated cytosine-phosphate-guanosine (CpG) DNA motifs through toll-like receptor 9, and we found that the synthetic CpG oligonucleotide 2006 (CpG) reduced the frequency of quiescent ALDH+ MCL cells, induced terminal plasma cell differentiation, and limited tumor formation in vitro and in vivo. Treatment with CpG also significantly enhanced the activity of the proteasome inhibitor bortezomib that was associated with induction of the unfolded protein response. Our data suggest that CpG may target clonogenic and resistant ALDH+ cells as well as improve the activity of proteasome inhibitors in MCL.
Introduction
Mantle cell lymphoma (MCL) is an aggressive, incurable B-cell malignancy that makes up 5% to 10% of non-Hodgkin lymphoma (NHL) cases.1 Patients with MCL typically present with extensive lymph node involvement as well as extranodal dissemination within the spleen, bone marrow, and gastrointestinal tract. Treatment with conventional cytotoxic agents produces high initial response rates, but the outcome of patients with MCL remains among the poorest of all NHL subtypes with a median overall survival of 3 to 4 years.2,3 Relapse after initial disease control suggests that a subset of cells can survive treatment and mediate tumor regrowth. In many cancers, specific populations of tumor cells with increased clonogenic potential have been identified and referred to as tumor initiating cells or cancer stem cells (CSCs).4 Similar to normal adult stem cells, CSCs may be quiescent and resistant to a wide variety of cytotoxic agents.5-7 However, few strategies have been developed to overcome CSC quiescence and chemoresistance.
Normal B cells can be activated by antigen binding to the B-cell receptor as well as several antigen-independent processes. Toll-like receptors (TLRs) are innate immune receptors that recognize a diverse range of pathogen-derived microbial molecules, and several TLRs are expressed during normal B-cell development.8,9 In humans TLR9 is expressed in B cells and plasmacytoid dendritic cells and recognizes unmethylated cytosine-phosphate-guanosine (CpG) motifs that mimic bacterial or viral DNA to induce cellular activation and differentiation.10-12 TLR9 is also expressed in a wide variety of B-cell leukemias and lymphomas, but CpG oligonucleotides (ODNs) may have varying effects, depending on the specific malignancy.10,13-16 CpG ODNs have been clinically studied as agents to induce or augment antitumor immunity in several tumor types, including B-cell NHL.17,18 Although these trials have shown limited benefit, CpG ODNs have been relatively well tolerated, suggesting that they may be safely combined with other antitumor agents. We studied the effects of CpG ODNs in MCL cells and found that they activate a minor population of relatively quiescent cells with increased clonogenic potential. Furthermore, CpG ODNs induce plasmacytic differentiation of MCL cells and enhance sensitivity to the proteosome inhibitor bortezomib.
Methods
Patient samples, cell lines, and cell culture
Clinical specimens were obtained from patients with active MCL who granted informed consent in accordance with the Declaration of Helsinki as approved by the Johns Hopkins Medical Institutes Institutional Review Board. The human MCL cells lines Granta 519, Jeko-1, and Rec-1 were obtained from the German Collection of Microorganisms and Cell Cultures. Cells were cultured in complete media consisting of RPMI 1640, 2mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 10% fetal bovine serum. Clonogenic growth was evaluated by plating 1000 cells/mL in 1 mL of 1.2% methylcellulose, 30% fetal bovine serum, 1% bovine serum albumin, 10−4M 2-mercaptoethanol, and 2mM l-glutamine. Samples were plated in triplicate onto 35-mm2 tissue culture dishes and incubated at 37°C and 5% CO2. Colonies consisting of more than 40 cells were scored between 7 and 10 days with an inverted microscope. Serial replating was performed by washing plates with complete media and resuspending cells in the original volume of methylcellulose. Treatment studies used CpG ODN 2006 (phosphorothioate: TCGTCGTTTTGTCGTTTTGTCGTT) or control ODN (phosphorothioate: TGCTGCTTTTGTGCTTTTGTGCTT) dissolved in phosphate-buffered saline (InvivoGen). In preliminary experiments we did not detect significant differences in clonogenic recovery or activation marker expression at CpG ODN concentrations ranging from 1 to 10 μg/mL that have been used in normal B-cell studies. Therefore, CpG and control ODNs were used at 5 μg/mL as in a previous study of MCL.13 For drug studies cells were treated with 1nM dexamethasone (Sigma-Aldrich), 1nM daunorubicin (Sigma-Aldrich), 0.1nM etoposide (Sigma-Aldrich), or 1 to 100nM bortezomib (Millennium Pharmaceuticals).
Fluorescence-activated cell sorting and flow cytometry
The following monoclonal antibodies were used: CD20-allophycocyanin, CD138–fluorescein isothiocyanate (FITC), CD40-phycoerthrin (PE), CD19-allophycocyanin, CD5-PE, CD86-PE, human leukocyte antigen-DR–FITC (all antibodies from BD PharMingen). Aldehyde dehydrogenase (ALDH) activity was detected with the Aldefluor reagent (StemCell Technologies) according to the manufacturer's instructions. Control staining reactions consisted of isotypic antibodies or the addition of the specific ALDH inhibitor diethylaminobenzaldehyde. Apoptosis analysis was performed with annexin V (FITC) and propidium iodide (BD Biosciences) according to the manufacturer's instructions. Cells were analyzed with a FACSCalibur flow cytometer or isolated with a FACSAria cell sorter (BD Biosciences). For clinical specimens, peripheral blood mononuclear cells were isolated by density centrifugation (Ficoll-Paque; Pharmacia) then stained with the Aldefluor reagent and anti-CD19 and CD5 antibodies.
Cell cycle and proliferation analyses
Cells (2 × 106/mL) in complete media were treated with 10μM bromodeoxyuridine (BrdU; BD Biosciences) for 15 minutes then washed in complete media and stained with the Aldefluor reagent as above. ALDH+ and ALDHneg cells were isolated by fluorescence-activated cell sorting, then fixed, and stained using the BrdU Flow Kit (BD Biosciences) according to the manufacturer's protocol. Metabolic activity was measured by MTT [3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide] analysis (Roche) according to the manufacturer's protocol.
Transplantation of MCL cells into nonobese diabetic/severe combined immunodeficient interleukin-2γ receptor−/− mice
All animal studies were approved by the Johns Hopkins Medical Institutes Animal Care Committee and were conducted in accordance with policy of the Institutional Animal Care and Use Committee. Nonobese diabetic/severe combined immunodeficient interleukin-2γ receptor−/− mice were bred and maintained in the Johns Hopkins animal core facility. Jeko-1 cells (1 × 106/animal) were injected into the tail vein of 6- to 10-week-old mice. Mice were observed daily and killed after the development of symptoms, including severely hunched posture, limited mobility, or both. Engraftment was detected by flow cytometric analysis of the spleen for human CD20+CD45+ cells.
Reverse transcriptase polymerase chain reaction
RNA was isolated with RNAqueous (Ambion) and cDNA made with the use of Superscript II (Invitrogen) with random hexamers according to the manufacturer's protocol. For quantitative reverse transcriptase polymerase chain reaction (RT-PCR), Taqman real-time primer/probes sets (Applied Biosystems) were used to detect cDNA for Blimp1 (Hs00153357_m1), XBP1 (Hs00231936_m1), CHOP (Hs_00358796_g1), CD138 (Hs00174579_m1), CD19 (Hs00174333_m1), and β-actin (4352935E). PCR was performed with TaqMan Universal Master Mix (4304437; ABI) on an I-Cycler Real-Time PCR machine (Bio-Rad). Expression levels were normalized to β-actin and compared with the ΔΔCT method. For semiquantitative RT-PCR, cDNA was amplified for 35 cycles [94°C for 30 seconds; 58°C for 30 seconds; and 72°C for 30 seconds (7 minutes in the final cycle)]. Using primers for TLR9 (forward, 5′-GTGCCCCACTTCTCCATG, and reverse, 5′-GGCACAGTCATGATGT TGT TG) and XBP1 spliced and unspliced variants (forward, 5′-CTGGAAAGCAAGTGGTAGA, and reverse, 5′-CTGGGTCCTTCTGGGTAGAC). PCR products representing spliced (XBP1S, 398 base pairs) and unspliced (XBP1U, 424 base pairs) were detected by agarose gel electrophoresis.
Statistical analysis
Results are presented as the mean ± SEM. Comparisons between groups were performed with a 2-tailed, paired Student t test. Kaplan-Meier analysis was carried out with a log-rank test. P values less than .05 were considered significant.
Results
Clonogenic MCL cells can be distinguished by ALDH activity
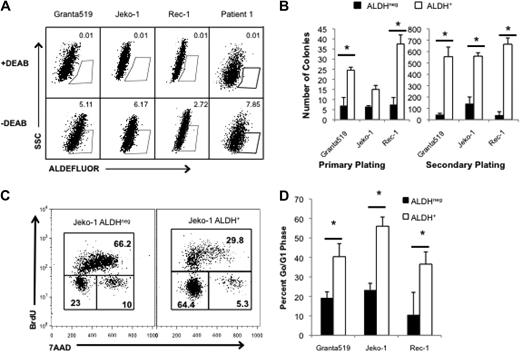
Highly tumorigenic CSCs can be identified in human cancers on the basis of specific cell-surface antigen expression or distinguishing functional properties. ALDH is a cytosolic enzyme required for the biosynthesis of all-trans-retinoic acid and is highly expressed by normal hematopoietic and neural stem cells.19,20 ALDH activity can also identify CSCs in breast, colon, and pancreatic carcinomas,21-23 and we previously demonstrated that it is expressed by tumorigenic cells in the B-cell malignancies multiple myeloma and classical Hodgkin lymphoma.24,25 We examined ALDH activity in 3 human MCL cell lines, Granta 519, Jeko-1, and Rec-1 with the use of the fluorescent ALDH substrate, Aldefluor, and found that each line contained a small population of ALDH+ cells (0.5%-8% of total cells; Figure 1A). To determine the relationship between ALDH expression and clonogenic potential, we isolated ALDH+ and ALDHneg cells from each cell line and plated them in methylcellulose to evaluate colony formation. In each cell line, ALDH+ MCL cells formed more colonies than ALDHneg cells (2.3- to 5-fold; Figure 1B). Furthermore, increased clonogenic potential was maintained during serial replating, suggesting that ALDH+ MCL cells have increased self-renewal capacity (Figure 1B; P < .05). We also examined ALDH expression in 4 distinct clinical MCL specimens and found that each contained ALDH+ tumor cells, ranging from 0.40% to 7.85% of all cells (Table 1).
MCL cells with the highest ALDH activity have enhanced clonogenic capacity and are more quiescent than bulk cells. (A) ALDH analysis of the human MCL lines Granta 519, Jeko-1, and Rec-1 and a representative clinical specimen by flow cytometry. ALDH+ populations are defined by gating for live cells by forward side scatter and in parallel with diethylaminobenzaldehyde (DEAB)–treated controls. Positive gates are determined by DEAB-treated cells and set at 0.01% for DEAB controls. For the patient samples, MCL tumor cells were initially identified by gating for CD19+CD5+ cells. Values represent percentage within each gate. (B) Colony formation by 1000 ALDH+ or ALDHneg cells. (C) Cell cycle analysis of ALDH+ and ALDHneg Jeko-1 cells. Values represent percentage within each gate. (D) Frequency of ALDH+ and ALDHneg cells in G0/G1 (*P < .05; n = 3). SSC indicates side scatter; 7AAD indicates 7-amino-actinomycin D.
MCL cells with the highest ALDH activity have enhanced clonogenic capacity and are more quiescent than bulk cells. (A) ALDH analysis of the human MCL lines Granta 519, Jeko-1, and Rec-1 and a representative clinical specimen by flow cytometry. ALDH+ populations are defined by gating for live cells by forward side scatter and in parallel with diethylaminobenzaldehyde (DEAB)–treated controls. Positive gates are determined by DEAB-treated cells and set at 0.01% for DEAB controls. For the patient samples, MCL tumor cells were initially identified by gating for CD19+CD5+ cells. Values represent percentage within each gate. (B) Colony formation by 1000 ALDH+ or ALDHneg cells. (C) Cell cycle analysis of ALDH+ and ALDHneg Jeko-1 cells. Values represent percentage within each gate. (D) Frequency of ALDH+ and ALDHneg cells in G0/G1 (*P < .05; n = 3). SSC indicates side scatter; 7AAD indicates 7-amino-actinomycin D.
ALDH+ cells in CD19+CD5+ primary MCL cells
| Patient no. . | ALDH+, % . |
|---|---|
| 1 | 7.85 |
| 2 | 0.90 |
| 3 | 1.20 |
| 4 | 0.40 |
| Patient no. . | ALDH+, % . |
|---|---|
| 1 | 7.85 |
| 2 | 0.90 |
| 3 | 1.20 |
| 4 | 0.40 |
Percentage of ALDH+ tumor cells from 4 patients with MCL. Cells were gated according to forward and side scatter followed by CD19+CD5+ coexpression for analysis of tumor cells.
ALDH+ MCL cells are relatively drug resistant
CSCs are relatively resistant to standard treatments, including radiation and chemotherapy.7,26,27 Therefore, they may persist after treatment and mediate disease relapse. To determine whether ALDH+ MCL cells are relatively drug resistant, we treated Granta 519, Jeko-1, and Rec-1 cells with dexamethasone, etoposide, or bortezomib, 3 commonly used therapeutic agents in MCL that have diverse mechanisms of action. After 48 hours of treatment at concentrations that induced 30% to 50% apoptosis within the bulk cultures, the number of absolute ALDH+ cells remained unchanged, and the frequency of ALDH+ cells increased in each cell line as bulk cells underwent apoptosis (Table 2). Therefore, ALDH+ cells are relatively resistant to standard chemotherapeutic agents compared with bulk ALDHneg cells.
Enrichment of ALDH+ cells after treatment
| . | Granta 519 . | Jeko-1 . | Rec-1 . |
|---|---|---|---|
| Dexamethasone | 2.09 ± 0.79 | 3.18 ± 0.09* | 4.01 ± 1.37 |
| Etoposide | 1.32 ± 0.22 | 1.49 ± 0.35 | 2.29 ± 1.12 |
| Bortezomib | 1.20 ± 0.13 | 2.14 ± 0.60 | 1.98 ± 0.55 |
| . | Granta 519 . | Jeko-1 . | Rec-1 . |
|---|---|---|---|
| Dexamethasone | 2.09 ± 0.79 | 3.18 ± 0.09* | 4.01 ± 1.37 |
| Etoposide | 1.32 ± 0.22 | 1.49 ± 0.35 | 2.29 ± 1.12 |
| Bortezomib | 1.20 ± 0.13 | 2.14 ± 0.60 | 1.98 ± 0.55 |
Fold change in the proportion of ALDH+ cells after 48 hours of treatment with dexamethasone (1nM), etoposide (0.1nM), or bortezomib (10nM) relative to vehicle-treated cells. Values represent the mean ± SEM from 4 independent experiments in each cell line. Aldefluor+ populations were defined by diethylaminobenzaldehyde control–treated cells for all samples.
P < .01.
Although ALDH can directly neutralize active metabolites of the cytotoxic alkylator cyclophosphamide,28,29 its expression does not explain resistance to all of the agents studied. Other cellular properties mediating drug resistance by both normal and cancer stem cells include expression of membrane-bound drug efflux pumps and cellular quiescence.5-7,19,20,30 To investigate whether putative ALDH+ MCL CSCs are more quiescent than bulk ALDHneg tumor cells, we performed cell cycle analysis of isolated ALDH+ and ALDHneg Granta 519, Jeko-1, and Rec-1 cells. ALDH+ Jeko-1 cells were significantly more quiescent than ALDHneg cells as evidenced by a 3-fold increase in the frequency of BrdUneg7AADneg cells representing the G0/G1 quiescent fraction (Figure 1C). Similarly, a significantly higher frequency of Granta 519 and Rec-1 ALDH+ cells were in G0/G1 compared with ALDHneg cells (Figure 1D; P < .05). Therefore, cellular quiescence may contribute to the relative drug resistance of clonogenic ALDH+ MCL cells.
MCL cell lines are activated by TLR9 stimulation
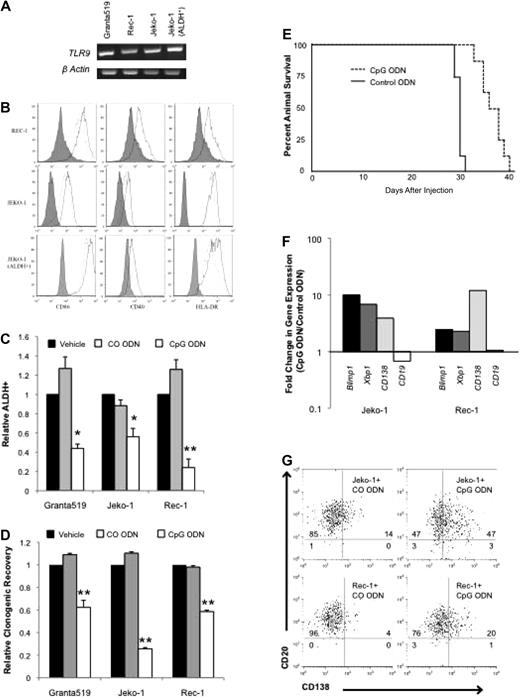
Normal B-cell activation and differentiation may be induced in an antigen-independent manner by virally or bacterially derived unmethylated CpG motifs through TLR9. We evaluated TLR9 expression in the MCL lines by RT-PCR and found that it was expressed in all 3 similar to previous findings as well as in ALDH+ cells isolated from the Jeko-1 cell line (Figure 2A).14 The synthetic unmethylated phosphorothioate CpG ODN 2006 has been found to induce activation of both normal and malignant B cells,11-15,31 and treatment of Granta 519, Jeko-1, and Rec-1 cells for 48 hours significantly increased the expression of the activation markers CD40, CD86, and human leukocyte antigen-DR by 1.2- to 3.0-fold compared with a scrambled control ODN (CO ODN) (Figure 2B; P < .01). Significant up-regulation of activation markers was also detected in Jeko-1 ALDH+ cells (Figure 2B; P < .01).
CpG ODN 2006 activates MCL cell lines and induces plasma cell differentiation. (A) RT-PCR for TLR9 expression in MCL cell lines and sorted ALDH+ Jeko-1 cells. (B) Expression of CD86, CD40, and human leukocyte antigen–DR after treatment with CO ODN (dashed lines), CpG (solid lines). Isotypic control staining is represented by the shaded peaks. (C) Frequency of ALDH+ cells after treatment with phosphate-buffered saline (PBS) vehicle, CO ODN, or CpG ODN (*P < .05, **P < .01; n = 3). (D) Relative clonogenic recovery after treatment with PBS vehicle, CO ODN, or CpG ODN (**P < .01; n = 3). (E) Survival of nonobese diabetic/severe combined immunodeficient interleukin-2γ receptor−/− mice after injection with CO (dashed line) or CpG (solid line) ODN–treated cells (P < .001; n = 8 per group). (F) Quantitative RT-PCR for BLIMP1, XBP1, CD138, and CD19 after CO ODN or CpG ODN treatment. Data represent fold change in expression relative to CO ODN–treated values. (G) CD138 and CD19 expression by flow cytometry after treatment with CO ODN or CpG ODN. Values represent percentage in each quadrant.
CpG ODN 2006 activates MCL cell lines and induces plasma cell differentiation. (A) RT-PCR for TLR9 expression in MCL cell lines and sorted ALDH+ Jeko-1 cells. (B) Expression of CD86, CD40, and human leukocyte antigen–DR after treatment with CO ODN (dashed lines), CpG (solid lines). Isotypic control staining is represented by the shaded peaks. (C) Frequency of ALDH+ cells after treatment with phosphate-buffered saline (PBS) vehicle, CO ODN, or CpG ODN (*P < .05, **P < .01; n = 3). (D) Relative clonogenic recovery after treatment with PBS vehicle, CO ODN, or CpG ODN (**P < .01; n = 3). (E) Survival of nonobese diabetic/severe combined immunodeficient interleukin-2γ receptor−/− mice after injection with CO (dashed line) or CpG (solid line) ODN–treated cells (P < .001; n = 8 per group). (F) Quantitative RT-PCR for BLIMP1, XBP1, CD138, and CD19 after CO ODN or CpG ODN treatment. Data represent fold change in expression relative to CO ODN–treated values. (G) CD138 and CD19 expression by flow cytometry after treatment with CO ODN or CpG ODN. Values represent percentage in each quadrant.
CpG depletes MCL ALDH+ cells and reduces clonogenic growth
We studied the effects of CpG treatment on ALDH+ cells within the Granta 519, Jeko-1, and Rec-1 cell lines and found that the frequency of these cells was significantly decreased (1.7- to 4.5-fold; P < .05) compared with vehicle or CO ODN (Figure 2C). In addition, we treated cells with vehicle, CpG, or CO ODN for 48 hours, then washed cells to remove drugs, and plated them in methylcellulose. In all 3 cell lines, CpG treatment significantly inhibited colony formation by 40% to 75% compared with the phosphate-buffered saline vehicle or CO ODN (Figure 2D; P < .01). Therefore, the loss of ALDH+ cells is associated with a decrease in clonogenic growth in vitro. We also studied effects on in vivo clonogenic growth potential by treating Jeko-1 cells in vitro with either CpG or CO ODN for 48 hours, then washing and injecting them intravenously into nonobese diabetic/severe combined immunodeficient interleukin-2γ receptor−/− mice. Although all mice showed evidence of engraftment, those receiving CpG-treated cells lived significantly longer than animals injected with CO ODN–treated cells (P < .01; Figure 2E). Therefore, activation of MCL cells by CpG results in the inhibition of clonogenic MCL growth both in vitro and in vivo.
We examined whether CpG inhibited clonogenic MCL growth by inducing cell death or inhibiting proliferation, but we found no differences in cell number, MTT activity, or Annexin V staining compared with CO ODN–treated cells after 48 hours, consistent with a previous report (data not shown).13 In normal tissues, clonogenic growth potential is inversely related to differentiation status. TLR9 signaling has been shown to induce the maturation of normal B cells into plasma cells12 ; therefore, we examined the differentiation state of CpG-treated MCL cells. Normal plasma cell differentiation is coordinated through well-characterized changes in gene expression induced by the transcription factors XBP1 and BLIMP1.32,33 CpG treatment of the Jeko-1 and Rec-1 cell lines induced the expression of both these genes as measured by quantitative RT-PCR (Figure 2F). In addition, CpG inhibited CD19 expression and induced CD138 expression at the mRNA and protein levels similar to normal plasmacytic maturation (Figure 2F-G).34 Taken together these data suggest that CpG depletes the clonogenic ALDH+ population by inducing plasmacytic differentiation.
CpG induces the unfolded protein response in MCL and enhances sensitivity to proteasome inhibition
Increased protein misfolding that accompanies enhanced immunoglobulin production during plasma cell differentiation results in endoplasmic reticulum stress and initiation of the unfolded protein response (UPR). As a result, the UPR limits general protein production and induces protein refolding and degradation activities.35 If the UPR fails to reduce the level of accumulated misfolded proteins, apoptosis is ultimately induced. Because plasma cell differentiation is associated with increased endoplasmic reticulum stress and UPR, we speculated that CpG ODN treatment might induce UPR in MCL. After 48 hours of CpG treatment Jeko-1 and Rec-1 cells displayed higher levels of total and the alternatively spliced variant of XBP1 (XBP1s), a well-established initiator of the UPR, thus confirming the induction of UPR by CpG ODN (Figure 2F and Figure 3A).
CpG ODN enhances the UPR and sensitivity to bortezomib. (A) RT-PCR for XBP1 spliced and unspliced (398- and 424-base pair fragments, respectively) forms in CpG ODN–treated Jeko-1 cells with and without bortezomib. (B) Relative clonogenic recovery of cells treated with CO or CpG ODN with or without bortezomib (*P < .05, **P < .01; n = 4). (C) Relative clonogenic recovery of Jeko-1 cells treated with CO or CpG ODN with or without bortezomib (10nM), daunorubicin (1nM), dexamethasone (1nM), or etoposide (0.1nM) or vehicle control (*P < .01; n = 3). (D) Quantitative RT-PCR for CHOP in Jeko-1 cells after treatment with phosphate-buffered saline vehicle, CO, or CpG ODN and bortezomib (**P < .02; n = 3).
CpG ODN enhances the UPR and sensitivity to bortezomib. (A) RT-PCR for XBP1 spliced and unspliced (398- and 424-base pair fragments, respectively) forms in CpG ODN–treated Jeko-1 cells with and without bortezomib. (B) Relative clonogenic recovery of cells treated with CO or CpG ODN with or without bortezomib (*P < .05, **P < .01; n = 4). (C) Relative clonogenic recovery of Jeko-1 cells treated with CO or CpG ODN with or without bortezomib (10nM), daunorubicin (1nM), dexamethasone (1nM), or etoposide (0.1nM) or vehicle control (*P < .01; n = 3). (D) Quantitative RT-PCR for CHOP in Jeko-1 cells after treatment with phosphate-buffered saline vehicle, CO, or CpG ODN and bortezomib (**P < .02; n = 3).
Proteasome inhibitors, such as bortezomib, may limit the degradation of misfolded proteins; thus, tumor cells that produce high levels of secreted proteins, such as multiple myeloma, are thought to be especially sensitive to these agents.36 The addition of bortezomib to CpG ODN–treated cells significantly reduced colony formation in all 3 lines compared with CpG alone or the combination of bortezomib and CO ODN (Figure 3B; P < .05). Moreover, treatment of ALDH+ Jeko-1 cells was also significantly inhibited by the combination of CpG ODN and bortezomib (Figure 3B; P < .01). This effect appeared to be specific to bortezomib because dexamethasone, daunorubicin, and etoposide failed to enhance the effect of CpG on the colony-forming potential of Jeko-1 cells (Figure 3C).
Recent studies in multiple myeloma have shown that bortezomib can induce the terminal, proapoptotic phase of the UPR marked by the expression of CHOP, a C/EBPα-related transcription factor.37,38 We found that CHOP expression in Jeko-1 increased less than 2-fold in response to CpG after 48 hours. (Figure 3D). However, when cells were treated with the combination of CpG and bortezomib for 48 hours, CHOP expression was further and significantly increased more than 20-fold in both unsorted and ALDH+ Jeko-1 cells (Figure 3D; P < .01). These data suggest that CpG may induce MCL apoptosis in both the bulk and the putative CSC population through the terminal phase of UPR and increase sensitivity to bortezomib.
Discussion
Several approaches have been used to distinguish tumorigenic CSCs from bulk tumor cells. In some diseases, surface markers expressed by normal tissue-specific stem cells can identify CSCs, such as CD34 in myeloid leukemias and CD133 in brain tumors.39-41 In multiple myeloma, CD19+CD27+ cells resembling normal memory B cells are capable of self-renewal and producing CD138+ plasma cells that characterize this disease.7 These data suggest that CSCs may be identified with the use of antigens expressed by normal self-renewing cells within a specific tissue. For mature pre-germinal center B-cell malignancies, such as MCL, this approach is limited because antigens that mark B cells capable of self-renewal are not available. Alternative methods to identify CSCs have taken advantage of functional properties common to normal adult stem cells. ALDH expression can identify tumorigenic cells in both solid tumors as well as the B-cell malignancies multiple myeloma and classic Hodgkin lymphoma.7,21,25,26 In this study, we similarly found that ALDH expression marks MCL cells with increased clonogenic potential.
In several diseases, CSCs have been found to be relatively drug resistant compared with their differentiated progeny, and these findings have suggested that their persistence after treatment is responsible for disease relapse.7,26,42 Several mechanisms are thought to promote drug resistance, including high expression of membrane-bound drug transporters and intracellular detoxification enzymes, such as ALDH.28,29 Although ALDH can directly neutralize active metabolites of cyclophosphamide and ifosfamide, we found that ALDH+ MCL cells were pan-resistant to agents with distinct mechanisms of action. CSCs have been found to be relatively quiescent in breast cancer, myeloid leukemias, and multiple myeloma,5-7,43 and this property may be protective in several ways. Many anticancer drugs, such as antimitotic agents, require actively cycling cells for maximal efficacy. Moreover, generally low transcriptional and metabolic activity may affect the expression of proteins inhibited by targeted therapies. In addition to these potential mechanisms, our data suggest that clonogenic ALDH+ MCL cells may have reduced protein production that limits their sensitivity to bortezomib. Few strategies have been designed to target quiescent CSCs, but our data show that CpG induces cellular activation and inhibits clonogenic MCL growth.
In addition to the loss of long-term proliferative potential, CpG-induced differentiation also sensitized MCL cells to bortezomib. Several mechanisms have been proposed to explain the inhibitory effects of proteasome inhibition in MCL, including increased expression of the proapoptotic BH3-only protein NOXA and inhibition of nuclear factor-κB activity.44 Our data suggest that bortezomib may also induce the terminal apoptotic phase of the UPR similar to recent data in multiple myeloma.37,38 In a recent multicenter trial, bortezomib produced an overall response rate of 33% and a median time to progression of 6.2 months in patients with relapsed MCL.45,46 Our results suggest that the combination of CpG ODN and bortezomib will improve clinical response rates by enhancing sensitivity to bortezomib and possibly lead to long-term remissions by depleting the CSCs responsible for disease relapse.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank George Weiner of the University of Iowa for the kind gift of CpG and CO ODN in preliminary experiments and for helpful discussion of these results.
This work was supported by the National Institutes of Health (R01CA127574, P01CA015396, K23CA107040), the Gabrielle's Angel Foundation for Cancer Research, the Sidney Kimmel Foundation for Cancer Research, and the Goodwin Foundation.
National Institutes of Health
Authorship
Contribution: S.K.B. designed and performed experiments and prepared the manuscript; B.M., Q.W., and A.A.M. performed experiments; J.K. performed statistical analysis; and W.M. designed experiments and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William Matsui, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, CRB245, 1650 Orleans St, Baltimore, MD 21287; e-mail: matsuwi@jhmi.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal