All living organisms contain polyphosphate—a linear polymer of inorganic phosphate. Depending on where they are derived, the polymers exist in different lengths. In this issue of Blood, Smith and colleagues demonstrate that polyphosphates exert differential effects on blood clotting depending on polymer size.
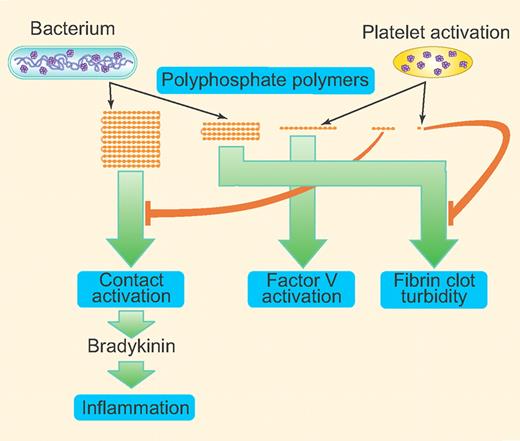
Inorganic polyphosphates (polyP), linear polymers of orthophosphate units linked by phosphoanhydride bonds, exist in all living organisms.1 In bacteria, polyP functions in basic metabolism and is important for growth and survival. However, the physiologic relevance of polyP in higher eukaryotes is unclear as has not been investigated to a great extent. Recent studies from Morrissey and colleagues show that polyP is a potent hemostatic regulator, affecting blood coagulation at various steps.2-5 PolyP-driven factor XII activation was shown to trigger the release of the inflammatory mediator bradykinin and cause bradykinin-mediated edema.5 The length of polyP polymer is known to vary greatly among different organisms and cell types, ranging from more than 1000 phosphate units in length in bacteria1 to as short as 60 phosphate units in length in human platelets.6 In this issue, Smith and colleagues report that polyP exerts differential effects on blood clotting depending on polymer length (summarized in figure).7 These findings suggest the possibility that polyP released by infecting bacteria affects the human body differently from that of polyP released in normal physiology by activation of platelets. More importantly, their findings raise an exciting possibility of developing a drug with a suitably size-fractionated polyP preparation to control bleeding without activation of the contact pathway and subsequent undesirable side effects.
Invading microorganisms release longer polyP polymers whereas activated platelets release shorter polymers (60-100 mer) or very short phosphates (pyrophosphate). Longer polymers (1000+) are very effective in activating the contact pathway of coagulation whereas shorter polymers (60-200 mer) are more effective in activating FV; ≥ 250 mer support maximal fibrin clot turbidity. Pyrophosphate released by platelets can block polyP-induced fibrin clot turbidity. Very short polymers (10-80 mer) can inhibit the contact pathway activation initiated by long-chain polyP. The polymers shown in the figure represent ∼ 1000+ mer, 250+ mer, 60-250 mer, 10-60 mer, and very short phosphates (mono-, pyro-, and triphosphates). The green arrows direct to the point in the clotting cascade that is shown to be affected maximally by the specific range of polymer size. (Professional illustration by Paulette Dennis.)
Invading microorganisms release longer polyP polymers whereas activated platelets release shorter polymers (60-100 mer) or very short phosphates (pyrophosphate). Longer polymers (1000+) are very effective in activating the contact pathway of coagulation whereas shorter polymers (60-200 mer) are more effective in activating FV; ≥ 250 mer support maximal fibrin clot turbidity. Pyrophosphate released by platelets can block polyP-induced fibrin clot turbidity. Very short polymers (10-80 mer) can inhibit the contact pathway activation initiated by long-chain polyP. The polymers shown in the figure represent ∼ 1000+ mer, 250+ mer, 60-250 mer, 10-60 mer, and very short phosphates (mono-, pyro-, and triphosphates). The green arrows direct to the point in the clotting cascade that is shown to be affected maximally by the specific range of polymer size. (Professional illustration by Paulette Dennis.)
Recent studies have established beyond a doubt that polyP is a potent modulator of blood coagulation and fibrinolysis. A series of studies from Morrissey and colleagues show that polyP acts at 3 different points in the blood clotting cascade: (1) triggering the contact pathway by activating factor XII; (2) accelerating the activation of factor V by thrombin and factor Xa; and (3) increasing the stability of fibrin clot by enhancing the thickness of fibrin fibers.2-5 In addition to modulating hemostasis, polyP is also shown to play a role in inflammation through activation of factor XII, which triggers the release of the inflammatory mediator bradykinin by plasma kallikrein-mediated kininogen processing.5 All previous studies on polyP were conducted with heterodisperse synthetic polyP or polyP purified from activated platelets. In the present study, Smith et al fractionated polyP preparations carefully to defined polymer lengths, from 30 mer to 1700 mer, and used them to investigate the effects of polyP on the blood-clotting system.7 Their data show that 30 mer does not activate the contact pathway. The procoagulant activity of polyP was detectable with a 53 mer, and thereafter the procoagulant activity increased as the length of polyP polymer increased. The strongest procoagulant activity was obtained with polyP1000+. An excess of short-chain polyP (30 mer to 80 mer), but not very short phosphates, inhibited plasma clotting initiated by long-chain polyP via the contact pathway. In contrast, maximal specific activities in FXa-initiated clotting were achieved with polyP polymers that were approximately 125 to 200 phosphates long. Similarly, shorter polyP polymers, ∼ 70 mer to 250 mer, are more effective in abrogating tissue factor pathway inhibitor–anticoagulant activity.
In the case of fibrin clot stabilization, polyP polymers shorter than 100 mers had no influence on fibrin clot turbidity whereas ≥ 250 mer support maximal turbidity increase. Interestingly, very short phosphates, particularly PPi, effectively block the polyP-mediated enhancement of clot turbidity. In most of the experiments, the authors have included polyP isolated from bacteria (mainly contain long polyP polymers) and platelets (contain short polymers, 60 mer to 100 mer), and their effects in the assays described above were consistent with their polymer length. Overall, these data support the authors' conclusion that short polyP polymers had very little ability to activate the contact pathway, but retain the ability to inhibit tissue factor pathway inhibitor–anticoagulant activity and accelerate FV activation. Based on these findings, exploring the possibility of developing short polyP polymers as pharmacologic agents to control bleeding without the unwanted side effect of systemic activation of the contact pathway is certainly worthwhile.
However, developing a shorter polymer as a potent procoagulant agent to control bleeding but not to have any side effects may not be as simple or straightforward. There is some overlap between shorter polyP polymers and longer polyP polymers in their procoagulant activity. Although this overlap may appear insignificant at face value, it still could pose a real problem in developing polyP as a valuable hemostatic agent. For example, even though the shorter polymers were 1000 times less effective than long polyP polymers in activating the contact pathway of blood coagulation, they are still 5-fold more potent than kaolin, an effective trigger of the contact pathway. This explains why polyP secreted from platelets, whose size ranges from 60 mer to 100 mer, exerts both procoagulant and proinflammatory activity in vivo.5 Although very short polyP polymers (< 50 mer) do not activate the contact pathway, they unfortunately fail to accelerate FV activation. Despite such limitations, it is important to conduct further studies, particularly in animal model systems, to investigate whether a polyP preparation could control bleeding without causing unwanted inflammation. For this to happen, in addition to formulating a precisely sized polyP polymer, precise dosage may have to be determined.
Success in exploiting the differential effects of differing length polyP polymers on blood clotting to develop a novel procoagulant agent would be a great boon to a vast number of patients with a variety of bleeding disorders. Currently, the primary factors limiting use of recombinant clotting factors, such as rFVIIa, as a universal hemostatic agent are high cost and potential liability associated with off-label use. Although the cost of rFVIIa could be expected to decrease in the future as patent restrictions expire, polyP may still be much less expensive.
Finally, the studies of Smith et al reported in this issue also identify a potential new role of platelet-released pyrophosphate, that is, as a novel modulator of fibrin clot structure as it blocks the effects of polyP on fibrin structure.7 The observation that short-chain polyP inhibits long-chain polyP-initiated activation of the contact pathway also deserves special attention. It would be interesting to investigate in the future whether short-chain polyP reduces the proinflammatory effect of long-chain polymers released by bacteria.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal