Abstract
The diagnosis of von Willebrand disease relies on abnormalities in specific tests of von Willebrand factor (VWF), including VWF antigen (VWF:Ag) and VWF ristocetin cofactor activity (VWF:RCo). When examining healthy controls enrolled in the T. S. Zimmerman Program for the Molecular and Clinical Biology of von Willebrand disease, we, like others, found a lower mean VWF:RCo compared with VWF:Ag in African American controls and therefore sought a genetic cause for these differences. For the African American controls, the presence of 3 exon 28 single nucleotide polymorphisms (SNPs), I1380V, N1435S, and D1472H, was associated with a significantly lower VWF:RCo/VWF:Ag ratio, whereas the presence of D1472H alone was associated with a decreased ratio in both African American and Caucasian controls. Multivariate analysis comparing race, SNP status, and VWF:RCo/VWF:Ag ratio confirmed that only the presence of D1472H was significant. No difference was seen in VWF binding to collagen, regardless of SNP status. Similarly, no difference in activity was seen using a GPIb complex-binding assay that is independent of ristocetin. Because the VWF:RCo assay depends on ristocetin binding to VWF, mutations (and polymorphisms) in VWF may affect the measurement of “VWF activity” by this assay and may not reflect a functional defect or true hemorrhagic risk.
Introduction
von Willebrand disease (VWD) is a common coagulation disorder, with a reported prevalence ranging from 0.01% to 1%.1,2 Defects in von Willebrand factor (VWF) are associated clinically with a spectrum of bleeding symptoms, including easy bruising, menorrhagia, and epistaxis, with affected patients at increased risk for excessive bleeding with invasive procedures. Type 1 VWD is characterized by mild quantitative defects of VWF, whereas type 3 VWD has virtually undetectable levels of VWF. The type 2 VWD variants are characterized by intrinsic functional defects, including defects in multimerization, platelet interactions, collagen binding, or factor VIII binding.3 Although type 1 VWD accounts for the majority of cases, the relationship between VWF levels and clinical symptoms is not always clear, particularly with modest decreases in VWF.3,4
The International Society on Thrombosis and Hemostasis has recommended provisional criteria for the diagnosis of type 1 VWD, which include a personal history of bleeding symptoms, the presence of affected family members, and laboratory findings consistent with the diagnosis.3 A panel of studies is recommended for workup of suspected VWD, including VWF antigen (VWF:Ag) as a measure of total VWF protein and VWF ristocetin cofactor activity (VWF:RCo) as a functional measure of VWF binding to platelets through their surface glycoprotein receptor GPIb. The VWF:RCo assay exploits the capacity of ristocetin to induce a conformational change in VWF that leads to VWF binding to platelet GPIb, but high variability and suboptimal sensitivity compromise the clinical utility of VWF:RCo.5-7 Many factors can influence VWF levels. ABO blood group is a known modifier, with the lowest VWF levels present in those with blood group O.8 A disproportionate number of patients diagnosed with type 1 VWD have blood group O.8,9 Race is another potential modifier. Miller et al demonstrated higher VWF:Ag levels in African American women compared with Caucasian women, even when controlled for blood type.10,11 Furthermore, VWF:RCo levels were similar between Caucasian and African American subjects, but the VWF:RCo/VWF:Ag ratios were lower for African Americans, attributed by the authors to their relative increase in VWF:Ag.
VWF binding to platelet GPIb occurs via a binding site in the VWF A1 domain.12,13 Type 2M VWD is characterized by a defective interaction between VWF and platelet GPIb in the absence of a significant defect in multimer structure.3 Type 2M VWD is clinically suspected when the VWF:RCo/VWF:Ag ratio is decreased, and has also been linked to mutations in the A1 domain.14-16 These mutations have been compiled online through the International Society on Thrombosis and Hemostasis Scientific and Standardization Committee VWF Online Database (www.VWF.group.shef.ac.uk).17 In the local population of patients with VWD followed at the Comprehensive Center for Bleeding Disorders in Milwaukee, African Americans were overrepresented in the subgroup of VWD patients with type 2M VWD. The demographics of our type 1 VWD patients are similar to our overall patient population, with 7% identified as African American. Our type 2M VWD patients, however, had a disproportionate number of African Americans, at 35%. Only 20% of Caucasians in our population with type 2 VWD were categorized as type 2M, whereas 80% of African American type 2 patients were classified as type 2M.18 Many of the latter group, however, had minimal bleeding symptoms despite a significantly reduced VWF:RCo/VWF:Ag ratio. We noted that a group of single nucleotide polymorphisms (SNPs) in exon 28 appeared frequently in the African American controls. These findings suggested the hypothesis that common polymorphisms in the A1 loop of VWF might affect the ristocetin-based activity assay and therefore lead to altered VWF:RCo/VWF:Ag ratios. To further investigate this hypothesis, we used data from control subjects in the T. S. Zimmerman Program for the Molecular and Clinical Biology of von Willebrand Disease (ZPMCB-VWD), a large, multicenter study of patients with VWD and healthy controls.
Methods
Patient population
Subjects were enrolled in the ZPMCB-VWD as healthy controls, with no previous diagnosis of a bleeding disorder. Exclusion criteria included a previous diagnosis of VWD and known pregnancy. Subjects were recruited from the local population, including hospital workers and community members, at 8 primary clinical centers in Atlanta, Detroit, Houston, Indianapolis, Iowa City, Milwaukee, New Orleans, and Pittsburgh. All healthy controls were 18 years of age or older. The study was approved by the Human Research Review Board at each institution and informed consent obtained from all subjects in accordance with the Declaration of Helsinki. Blood was collected for further testing as detailed below.
A bleeding questionnaire was administered to all subjects, including controls. Questions included those used in the European Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWD study19 as well as a set of additional questions specific to the ZPMCB-VWD. Bleeding scores for this manuscript were calculated using the European Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWD scoring system.19 Only controls with completed bleeding scores, laboratory testing, and gene sequencing results are included in this analysis.
DNA analysis
Polymerase chain reaction (PCR) amplification and semiautomated bidirectional sequencing using dye-terminator chemistry was performed using a set of primers designed specifically to amplify the entire coding sequence of VWF, including intron-exon boundaries, 3.5 kb upstream of exon 1, and 1 kb downstream of the C-terminal stop codon while excluding the pseudogene sequence. Primer sequences and PCR conditions are available on request. To examine possible linkage of the 3 SNPs of interest on 1 allele, PCR of exon 28 was performed on 3 African American subjects and the resulting fragment cloned into the TopoTA cloning vector (Invitrogen). Exon 28 sequencing was then performed on purified DNA from the resulting colonies.
VWF testing
VWF:Ag was measured in the clinical laboratory at BloodCenter of Wisconsin by an enzyme-linked immunosorbent assay using 2 monoclonal antibodies (AVW-1 and AVW-5) for capture and an horseradish peroxidase-conjugated rabbit polyclonal anti-VWF antibody for detection (Dako North America). VWF:RCo was also measured in the clinical laboratory using the Dade Behring BCS System and a reagent containing lyophilized platelets and ristocetin purchased from the manufacturer (Dade Behring, Siemens Healthcare Diagnostics). The final ristocetin concentration was 1 mg/mL. A standard plasma calibrated against an international reference plasma was used as a control. VWF multimers were assessed on 0.65% agarose multimer gels as previously described.20 Collagen binding was measured using type III human placental collagen plated at a concentration of 1 μg/mL (Southern Biotechnology Associates) for capture and a rabbit polyclonal antibody for detection (Dako North America). FVIII activity was measured using a 1-stage clotting assay.8 Blood group was determined by testing plasma samples for the presence of isohemagglutinins. A subset of samples was sent to 5 additional laboratories (ARUP Laboratories, Centers for Disease Control, Esoterix, Mayo Clinic, and Queen's University, Kingston, ON) for VWF:Ag and VWF:RCo testing to confirm the results obtained.
Platelet aggregation studies
Platelet aggregation was performed in the BloodCenter of Wisconsin clinical hemostasis laboratory using fresh platelet-rich plasma diluted to a platelet count of 300 000/μL. Ristocetin was added at concentrations ranging from 0.5 to 1.5 mg/mL and absorbance measured on a Chronolog platelet aggregometer for a minimum of 5 minutes.
Botrocetin binding assay
Fixed lyophilized reconstituted platelets (Bio/Data) were incubated with various concentrations of botrocetin (0-1 μg/mL) and plasma from African American subjects either homozygous for the 1472H allele or homozygous for the 1472D allele. After incubation for 1 hour, a polyclonal anti-VWF antibody (Dako North America) conjugated to Alexa Fluor 633 (Invitrogen) was added and fluorescence measured as previously described.21
GPIb complex-binding assay
A construct containing full-length GPIbα was made with 2 gain-of-function mutations, G233V and M239V, and expressed in HEK293T cells together with GPIbβ and GPIX to improve surface GPIb expression. Additional constructs were made containing a novel GPIbα mutation, D235Y, in combination with either G233V or M239V, as well as a construct containing all 3 mutations. Constructs containing any 2 mutations demonstrated greater reactivity, and the construct with G233V and M239V was used for further study. Cells were collected at 60 hours and equal numbers of cells aliquoted into each well of a 96-well plate. Plasma (collected in 3.2% sodium citrate) from ZPMCB-VWD healthy controls was added to the cells. A fluorescently labeled polyclonal antibody to VWF (Dako North America) was added and the resulting complex detected using an LSR II flow cytometer (BD Biosciences). A normal curve was constructed with lyophilized, reconstituted normal control plasma calibrated to the World Health Organization standard.
Statistics
The statistical program Stata (StataCorp LP) was used to perform all statistical analyses. Comparisons of mean VWF:Ag, VWF:RCo, and VWF:RCo/VWF:Ag ratios used 1-way and 2-way analysis of variance with individual comparisons using an analysis of variance-based t test after a normalizing transform. Comparisons within the same subjects used a paired t test (for comparison of the VWF:RCo/VWF:Ag with GPIb complex-binding/VWF:Ag). VWF:Ag and VWF:RCo values were log-transformed to achieve a normal distribution before analysis. Multiple comparison adjustments used the Tukey Honestly Significant Difference method. Multiple linear regression analysis was used to compare VWF:Ag, VWF:RCo, GPIb complex binding, race, and SNP status; in addition, the dominant and additive genetic models were examined for the relationship with the D1472H SNP.
Results
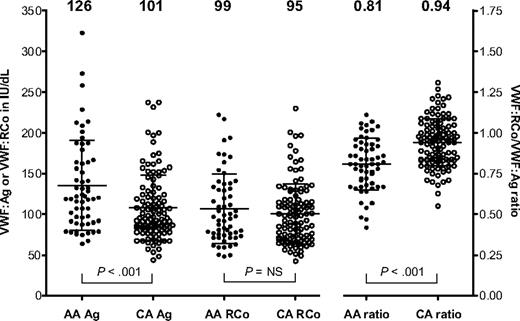
VWF:Ag, VWF:RCo, and VWF exon 28 gene sequencing results were examined for a group of healthy control subjects enrolled in the ZPMCB-VWD. The initial group of controls included 59 African Americans and 113 Caucasians. As illustrated in Figure 1, the mean VWF:Ag in the African American controls was 126 IU/dL, whereas the mean VWF:Ag for the Caucasian controls was 101 IU/dL. In contrast, the VWF:RCo levels were similar: 99 IU/dL for the African American controls and 95 IU/dL for the Caucasian controls. VWF:RCo/VWF:Ag ratios were lower in the African American controls, with a mean ratio of 0.81 compared with 0.94 for the Caucasian controls (Figure 1). These results are similar to those previously reported by Miller et al.10,11
Scatterplot of VWF:Ag (Ag), VWF:RCo (RCo), and VWF:RCo/VWF:Ag ratio (ratio) for all African American (AA; ●) and Caucasian controls (CA; ○) enrolled in the ZPMCB-VWD. The mean value for each assay is shown at the top of the graph for 59 African American and 113 Caucasian healthy controls. Error bars represent ± 1 SD.
Scatterplot of VWF:Ag (Ag), VWF:RCo (RCo), and VWF:RCo/VWF:Ag ratio (ratio) for all African American (AA; ●) and Caucasian controls (CA; ○) enrolled in the ZPMCB-VWD. The mean value for each assay is shown at the top of the graph for 59 African American and 113 Caucasian healthy controls. Error bars represent ± 1 SD.
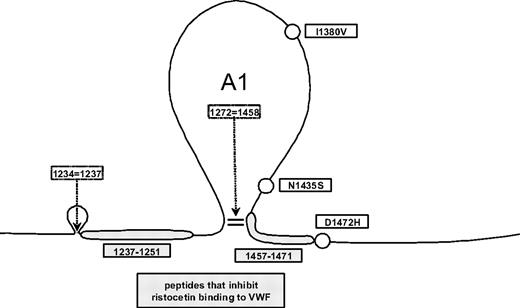
DNA sequencing results revealed a trio of SNPs (I1380V, N1435S, and D1472H) that appeared frequently in the African American controls, but rarely in the Caucasian controls. Cloning of exon 28 from 3 of the African American subjects with the 3 SNPs demonstrated that these SNPs were present on the same allele for all 3 subjects (data not shown). I1380V is located in the middle of the A1 loop. N1435S and D1472H are located just before and just after the C-terminal disulfide bond of the A1 loop (Figure 2). This 3-SNP haplotype was present in 22% of the African American controls, compared with only 3% of the Caucasian controls. An additional 39% of African American controls had the D1472H SNP alone. Whereas 63% of the African American controls were positive for the D1472H SNP, only 17% of Caucasians had this SNP (P < .001). The majority of the Caucasian controls (81%) had none of these SNPs (Table 1). No known type 2M mutations were observed in the control population. The mean VWF:RCo/VWF:Ag ratio in African American subjects with the 3-SNP haplotype was 0.71, whereas the mean VWF:RCo/VWF:Ag ratio in African American subjects with only D1472H was 0.77 (P = not significant). In contrast, the mean VWF:RCo/VWF:Ag ratio for the African American subjects with none of the SNPs in question was 0.91, significantly different from those with the 3-SNP haplotype and from those with D1472H alone (P < .001).
Common exon 28 SNPs found in the VWF A1 loop. Circles represent the approximate location of the 3 common SNPs, I1380V, N1435S, and D1472H, which were seen most frequently in the African American controls; gray boxes, known regions implicated in VWF-ristocetin interactions.
Common exon 28 SNPs found in the VWF A1 loop. Circles represent the approximate location of the 3 common SNPs, I1380V, N1435S, and D1472H, which were seen most frequently in the African American controls; gray boxes, known regions implicated in VWF-ristocetin interactions.
Characteristics of exon 28 SNPs in African American and Caucasian controls
| . | SNP status . | No. (%) . | Mean VWF:Ag, IU/dL . | Mean VWF:RCo, IU/dL . | Mean ratio of VWF:RCo/VWF:Ag . |
|---|---|---|---|---|---|
| African American | D1472H alone | 23 (39) | 122 | 93 | 0.77 |
| D1472H | 13 (22) | 135 | 93 | 0.71 | |
| I1380V | |||||
| N1435S | |||||
| No SNPs | 22 (37) | 124 | 112 | 0.91 | |
| Caucasian | D1472H alone | 19 (17) | 121 | 98 | 0.82 |
| D1472H | 3 (3) | 126 | 97 | 0.77 | |
| I1380V | |||||
| N1435S | |||||
| No SNPs | 91 (81) | 97 | 94 | 0.97 |
| . | SNP status . | No. (%) . | Mean VWF:Ag, IU/dL . | Mean VWF:RCo, IU/dL . | Mean ratio of VWF:RCo/VWF:Ag . |
|---|---|---|---|---|---|
| African American | D1472H alone | 23 (39) | 122 | 93 | 0.77 |
| D1472H | 13 (22) | 135 | 93 | 0.71 | |
| I1380V | |||||
| N1435S | |||||
| No SNPs | 22 (37) | 124 | 112 | 0.91 | |
| Caucasian | D1472H alone | 19 (17) | 121 | 98 | 0.82 |
| D1472H | 3 (3) | 126 | 97 | 0.77 | |
| I1380V | |||||
| N1435S | |||||
| No SNPs | 91 (81) | 97 | 94 | 0.97 |
I1380V and N1435S were only found in heterozygous form. All the Caucasian controls with D1472H were heterozygous for this SNP, whereas 35% of the African American controls with D1472H were homozygous for the H allele. One African American subject had I1380V and D1472H (not included in table).
SNP indicates single nucleotide polymorphism; VWF, von Willebrand Factor; Ag, antigen; IU, International Unit; and RCo, ristocetin cofactor.
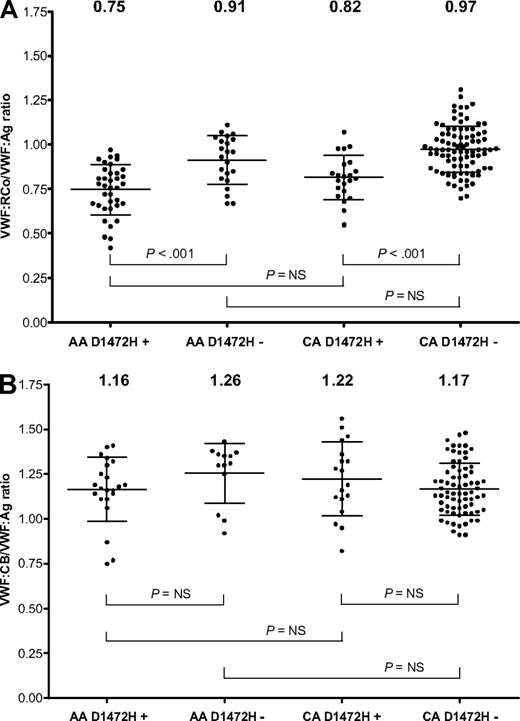
Because the D1472H SNP appeared relatively frequently in both the African American and Caucasian controls, this SNP was investigated separately (Figure 3A). The mean VWF:RCo/VWF:Ag ratio for African Americans with the D1472H SNP alone was 0.75, compared with 0.91 for those without the SNP (P < .001). For the Caucasian controls, the mean VWF:RCo/VWF:Ag ratio for those with D1472H was 0.82, compared with a ratio of 0.97 for those without the SNP (P < .001). There was no significant difference between African Americans and Caucasians with D1472H, nor was there a statistical difference between African Americans and Caucasians without D1472H. To confirm that these results were not simply the result of the VWF:RCo assay used by our laboratory, samples with and without the D1472H polymorphism were sent to 5 North American laboratories. Similar results to those obtained in our laboratory were again observed, with the D1472H samples yielding a lower ratio than the samples without this polymorphism. The average ratio across the 5 laboratories (as normalized to an international standard) for subjects with D1472H was 0.80, whereas the average ratio for the subjects without D1472H was 0.95. It is important to note that 2 of these laboratories perform the VWF:RCo assay using a similar test protocol to ours, whereas 3 laboratories use platelet aggregometry instruments.
VWF activity to antigen ratios by race and D1472H status. (A) VWF:RCo/VWF:Ag ratios for African American (AA) and Caucasian (CA) subjects with or without the D1472H polymorphism. (B) VWF:CB/VWF:Ag ratios for African American and Caucasian subjects with or without the D1472H polymorphism. The mean value for each group is listed at the top of the graph. Error bars represent ± 1 SD.
VWF activity to antigen ratios by race and D1472H status. (A) VWF:RCo/VWF:Ag ratios for African American (AA) and Caucasian (CA) subjects with or without the D1472H polymorphism. (B) VWF:CB/VWF:Ag ratios for African American and Caucasian subjects with or without the D1472H polymorphism. The mean value for each group is listed at the top of the graph. Error bars represent ± 1 SD.
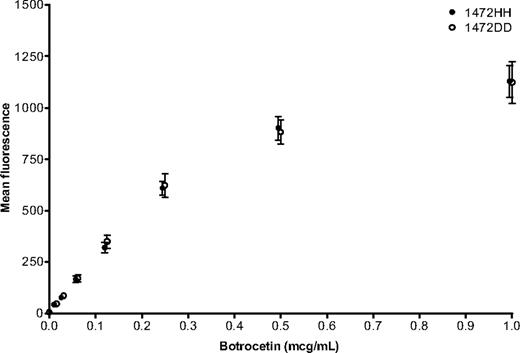
To determine whether the apparent decrease in function was the result of an isolated issue with the VWF:RCo assay or an actual defect in VWF function, VWF:collagen binding (VWF:CB) was examined. Although the major binding site for collagen is located in the VWF A3 domain, there is a collagen-binding site in the A1 domain that could potentially be disrupted by the D1472H polymorphism.22 Previous work showed an elevation in VWF:CB, similar to that seen with VWF:Ag, when African American controls were compared with Caucasians.11 When both African American and Caucasian controls from the ZPMCB-VWD were analyzed based on the presence or absence of D1472H, no significant difference in VWF:CB/VWF:Ag ratio was seen (Figure 3B). Therefore, the D1472H polymorphism appears to have minimal effect on VWF-collagen interactions. Botrocetin was also tested as an additional method of inducing VWF-platelet interactions. When 6 subjects homozygous for the 1472H allele were compared with 6 subjects homozygous for the 1472D allele, no difference was observed over a range of botrocetin concentrations (Figure 4). When bleeding scores were examined, the mean for the African American subjects with D1472H was −0.49 (range, −3 to 4), whereas the mean for African Americans without D1472H was 0.23 (range, −2 to 4). Similar results were observed for the Caucasian controls. In general, a bleeding score more than or equal to 4 is considered significant.19,23 Only 3 controls had a bleeding score more than 4, all Caucasians without the D1472H polymorphism.
Botrocetin-induced binding of VWF to platelets. African American subjects homozygous for 1472H (●) are compared with African American subjects homozygous for 1472D (○). Error bars represent ± SEM. 1472H points have been moved slightly to the left to allow better visualization of the data points (n = 6 for each group).
Botrocetin-induced binding of VWF to platelets. African American subjects homozygous for 1472H (●) are compared with African American subjects homozygous for 1472D (○). Error bars represent ± SEM. 1472H points have been moved slightly to the left to allow better visualization of the data points (n = 6 for each group).
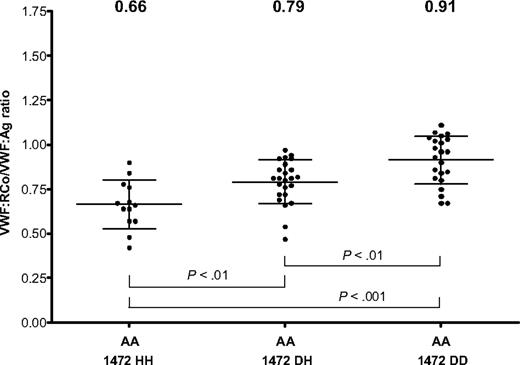
Although none of the Caucasians was homozygous for D1472H, there were a number of African American subjects homozygous for this SNP, allowing analysis of copy number effect based on number of 1472H alleles (Figure 5). The mean VWF:RCo/VWF:Ag ratio for the homozygous 1472H African American controls was 0.66, and the mean ratio for the heterozygous D1472H African American controls was 0.79 (P < .01). When the heterozygous D1472H African American controls were compared with those homozygous for the wild-type sequence, there was also a significant difference (P < .01). This suggests a dose effect of the 1472H variant in response to ristocetin in the African American population. Three of the African American controls had VWF:RCo/VWF:Ag ratios less than 0.5, and 3 more had ratios less than 0.6. None of these 6 subjects had elevated bleeding scores, although their VWF:RCo/VWF:Ag ratios do fall into the range of type 2M VWD. This is important, as a ratio of less than 0.6 has been proposed as an indicator of type 2 VWD.24,25 The majority (60%) of subjects enrolled in the ZPMCB-VWD to date with a diagnosis of type 2M VWD had bleeding scores more than or equal to 4 (mean, 4.4; range, of −2 to 13).
VWF:RCo/VWF:Ag ratio in African American (AA) controls by D1472H allele status. VWF:RCo/VWF:Ag ratios are shown for African American subjects homozygous for the 1472H allele (1472 HH), heterozygotes (1472 DH), and those homozygous for the 1472D allele (1472DD). The mean value for each group is listed at the top of the graph. Error bars represent ± 1 SD.
VWF:RCo/VWF:Ag ratio in African American (AA) controls by D1472H allele status. VWF:RCo/VWF:Ag ratios are shown for African American subjects homozygous for the 1472H allele (1472 HH), heterozygotes (1472 DH), and those homozygous for the 1472D allele (1472DD). The mean value for each group is listed at the top of the graph. Error bars represent ± 1 SD.
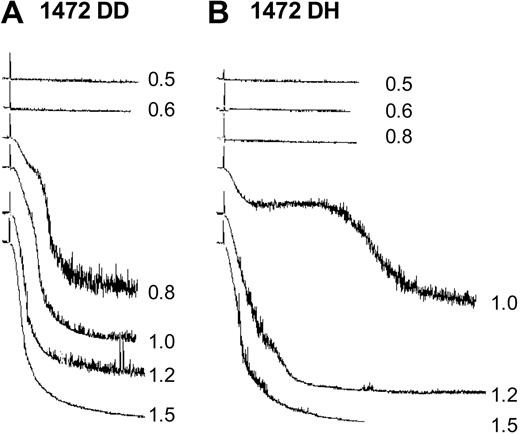
Response to various doses of ristocetin was assessed with platelet aggregation studies. Platelet-rich plasma from a subject heterozygous for the D1472H polymorphism was compared with a wild-type subject at doses of ristocetin ranging from 0.5 to 1.5 mg/mL. A marked difference was observed (Figure 6), with no aggregation at 0.8 mg/mL and delayed aggregation at 1 mg/mL for the D1472H subject compared with the normal control.
Ristocetin-induced platelet aggregation. Various concentrations of ristocetin were added to platelet-rich plasma from a control subject (A) and a subject heterozygous for the D1472H polymorphism (B).
Ristocetin-induced platelet aggregation. Various concentrations of ristocetin were added to platelet-rich plasma from a control subject (A) and a subject heterozygous for the D1472H polymorphism (B).
In the multiple linear regression analysis, the only significant finding was the presence of D1472H, after analysis for race, bleeding score, VWF:RCo/VWF:Ag ratio, and SNP status at I1380V, N1435S, and D1472H (P < .005). Although there were only a small number of homozygous D1472H subjects in the sample (n = 13), the genetic effect of the homozygous D1472H had slightly more than twice the effect on the ratio as the heterozygous (0.12 vs 0.26, P < .001). After accounting for the D1472H SNP, ethnicity had a marginal effect on the ratio (0.047, P = .052). Because the D1472H SNP accounted for essentially all of the variability in VWF:RCo/VWF:Ag ratios, it is probable that the association of decreased ratios and the 3 SNP haplotype occurs simply because D1472H is frequently linked in African Americans to the other 2 exon 28 polymorphisms.
To further investigate the effect of these polymorphisms on VWF-GPIb interactions independent of ristocetin, an assay was developed using GPIb mutations that allow spontaneous VWF binding even in the absence of ristocetin, referred to here as the GPIb complex-binding assay. The VWF:RCo/VWF:Ag ratio obtained using the conventional ristocetin-based assay was compared with the GPIb complex-binding/VWF:Ag ratio obtained with the new GPIb-VWF binding assay. Another common polymorphism in the VWF A1 domain, A1381T, has been previously reported to affect VWF-GPIb interactions, causing an increased affinity for GPIb.26 Therefore, we excluded those subjects with A1381T from analysis with our GPIb complex-binding assay, leaving 37 African American and 30 Caucasian controls with GPIb complex-binding results available for analysis.
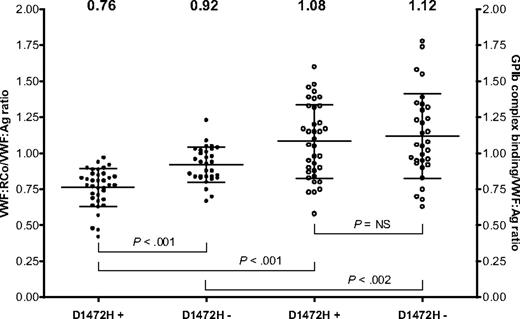
Unlike the results obtained with VWF:RCo/Ag ratios, no racial difference was seen in the GPIb complex-binding/VWF:Ag ratio. African American and Caucasian controls both had a mean GPIb complex-binding/VWF:Ag ratio of 1.10 (P = not significant). When subjects were divided according to race and SNP status at D1472H, no statistically significant difference in GPIb complex-binding/VWF:Ag ratio was observed, suggesting that this assay is not affected by the presence of the D1472H polymorphism. African American controls with the polymorphism had a mean ratio of 1.06, whereas those without the polymorphism had a mean ratio of 1.2. Caucasian controls with the polymorphism had a mean ratio of 1.13, whereas those without the polymorphism had a mean ratio of 1.08 (P = not significant for all comparisons). Several subjects with published type 2M VWD mutations (Δ1392-1402, G1324S, and I1425F) were tested using the GPIb complex-binding assay and showed markedly reduced binding (data not shown), confirming that this assay is sensitive to VWF-GPIb interactions that might cause type 2M VWD. Figure 7 shows VWF:RCo/VWF:Ag and GPIb complex-binding/VWF:Ag ratios for subjects with or without the D1472H polymorphism. Subjects with D1472H have GPIb complex-binding values that are higher than their VWF:RCo values, suggesting that the VWF:RCo assay may underestimate VWF-platelet binding with this polymorphism, at least compared with an assay that uses spontaneous VWF-GPIb binding instead of a ristocetin-mediated interaction. This effect is independent of race because similar results are seen in African American and Caucasian subjects. Neither race nor D1472H status had a significant effect on GPIb complex-binding/VWF:Ag ratios in a multiple linear regression analysis.
VWF:RCo assay compared with VWF GPIb complex-binding assay. The first 2 columns show VWF:RCo/VWF:Ag ratio (●) for subjects with and without the D1472H polymorphism. The second 2 columns show the GPIb complex-binding assay/VWF:Ag ratio (○) for subjects with and without the D1472H polymorphism. The mean value for each group is listed at the top of the graph. Error bars represent ± 1 SD.
VWF:RCo assay compared with VWF GPIb complex-binding assay. The first 2 columns show VWF:RCo/VWF:Ag ratio (●) for subjects with and without the D1472H polymorphism. The second 2 columns show the GPIb complex-binding assay/VWF:Ag ratio (○) for subjects with and without the D1472H polymorphism. The mean value for each group is listed at the top of the graph. Error bars represent ± 1 SD.
Discussion
Our results suggest that racial differences in VWF:RCo/VWF:Ag ratios are the result of increased frequency of the D1472H polymorphism in African Americans. This study is not the first to note racial differences in the dose response to added ristocetin. Ristocetin-induced platelet aggregation has been shown to be lower in African Americans compared with Caucasians, particularly with lower concentrations of ristocetin.27-29 African Americans had higher levels of VWF:Ag, with an associated decrease in the VWF:RCo/VWF:Ag ratio and a similar increase in VWF collagen binding compared with blood type–matched Caucasian controls.11 We propose here that racial differences in the prevalence of specific SNPs in the A1 domain may affect the VWF:RCo assay and therefore impact the VWF:RCo/VWF:Ag ratio in vitro, but may not necessarily affect in vivo function. The increased number of African American subjects with a low VWF:RCo/VWF:Ag ratio may simply reflect a higher prevalence of A1 domain SNPs that affect ristocetin-mediated binding. It should be noted, however, that the reason for the increase in VWF:Ag in this population remains to be determined.
It is possible that the D1472H SNP decreases ristocetin-mediated VWF-GPIb interactions, through alteration of the ristocetin-binding site on VWF. In support of this theory, control subjects in the ZPMCB-VWD study with D1472H had lower VWF:RCo/VWF:Ag ratios, on several occasions low enough to be considered for diagnosis of type 2M VWD, yet no increase in bleeding score was observed. In addition, 2 other SNPs in this region, I1380V and N1435S, were found to correlate with low VWF:RCo/VWF:Ag. These SNPs are linked to D1472H, and our data show that the latter SNP appears to predominate, as there is no significant difference between D1472H alone and the presence of the 3 SNPs together. The role of I1380V and N1435S remains undefined as we had no subjects with these SNPs in the absence of D1472H. This relationship may reflect linkage with D1472H or perhaps additional alterations in the A1 loop structure affecting ristocetin binding.
If the D1472H polymorphism affects in vitro ristocetin binding without affecting other VWF functions, other assays should show no difference between samples from subjects with and without this SNP. VWF:CB, when controlled for VWF:Ag, was not affected by D1472H SNP status; however, the collagen-binding site is separate from the GPIb-binding site.30 The GPIb complex-binding assay, however, provides another means of ristocetin-independent assessment of VWF-platelet interactions. There was no discernable difference in VWF-GPIb interactions, regardless of SNP status, as assessed by the GPIb complex-binding assay, which measures platelet GPIb binding independent of ristocetin. Furthermore, binding was similar for subjects with and without D1472H, regardless of race, and no difference in the GPIb complex-binding assay was seen for African American subjects with the 3-SNP haplotype compared with those without the 3 SNPs. These data suggest that the D1472H SNP may affect the VWF:RCo assay but might not cause a physiologically significant decrease in VWF function in vivo. Bleeding score data are entirely consistent with absence of physiologic consequences for the D1472H polymorphism.
Ristocetin causes enhanced platelet aggregation by inducing binding of VWF to platelet GPIb.31 Ristocetin was removed from clinical use as an antibiotic because of its proclivity for causing drug-induced thrombocytopenia32 but remains important in laboratory testing for VWD. When added to plasma, ristocetin binds VWF, facilitating VWF-GPIb binding in vitro and enabling a quantitative assessment of VWF “activity” by measuring platelet aggregation or agglutination.33,34 Ristocetin in the presence of plasma VWF significantly decreased platelet electrophoretic mobility, probably because of VWF-platelet interactions promoted by ristocetin.35 It can also precipitate plasma proteins, including both VWF and fibrinogen.31 VWF-GPIb interactions in vivo, however, occur under shear stress in proximity to damaged endothelium, and the ensuing conformational change in multimeric VWF might not necessarily mimic that which happens in response to ristocetin.
The interaction between VWF and platelet GPIb involves specific regions located in the A1 domain. The crystal structure of the VWF A1 domain in complex with platelet GPIb spans amino acids 1261 to 1468 of the VWF protein.36 Early studies localized 2 regions in or near the A1 loop (Cys 1237-Pro 1251 and Leu 1457-Pro 1471) as important in VWF interactions with platelet GPIb in the presence of ristocetin.37 Further studies using the synthetic peptides Cys 1237-Pro 1251 and Ser 1455-Pro 1471 were able to demonstrate inhibition of a substantial proportion of ristocetin-induced VWF-GPIb binding, confirming the importance of these regions in ristocetin-mediated interactions.38 A monoclonal antibody recognizing Glu 1463-Asp 1472 could also inhibit ristocetin-induced VWF binding to platelets.39 Recently, another A1 loop polymorphism, A1381T, was reported to result in increased affinity for GPIb because of an increased sensitivity to ristocetin but did not appear to affect GPIb-VWF interactions subjected to shear conditions.26 This may well be another example of an A1 domain polymorphism that is sensitive to the nonphysiologic use of ristocetin without altering in vivo VWF interactions.
The VWF:RCo assay as presently used is important in distinguishing the type 2 variants. Type 2M VWD is suspected based on a low VWF:RCo/VWF:Ag ratio with normal multimers. Expert guidelines have used a VWF:RCo/VWF:Ag ratio of less than 0.5 or less than 0.7 as diagnostic of type 2 VWD.3,25 In 2M VWD, as with the other type 2 VWD variants, bleeding symptoms are usually more severe than those seen in type 1 patients. It is important to distinguish polymorphisms that affect ristocetin interactions to avoid falsely labeling patients with 2M VWD when in reality they may actually have type 1 VWD (or possibly not meet diagnostic criteria for VWD at all).
Several 2M mutations are located close to the SNPs of interest. An 11-amino acid deletion involving Arg 1392-Gln 1402 was discovered in a family with mucocutaneous and postsurgical bleeding, low VWF:Ag, and disproportionately low VWF:RCo.15 Mutations at Phe 1369 and Ile 1425 in 2 unrelated families with type 2M VWD both lead to defective ristocetin-mediated binding of VWF to platelet GPIb and significant clinical bleeding.16 We have recently shown that the P1467S mutation causes decreased VWF:RCo but does not appear to be associated with any relevant clinical bleeding.21 Another example of VWD with abnormal ristocetin interactions is VWD type 2B Malmö, otherwise known as type 1 New York or type 1 Malmö. This variant, P1266L, is associated with increased aggregation in the presence of low-dose ristocetin.40,41 However, the absence of thrombocytopenia and normal distribution of VWF multimers are noteworthy distinctions between the P1266L variant and patients with other type 2B-associated mutations, such that one must wonder whether the results with ristocetin are of physiologic importance in this variant as well.
Given the wide range of VWF:Ag in the normal population, there is the potential for healthy persons to have VWF:RCo levels low enough to merit a diagnosis of VWD. In the presence of specific SNPs, the VWF:RCo/VWF:Ag ratio may lead to the classification of the patient as VWD type 2M, when in reality, the discrepancy between VWF:RCo and VWF:Ag may be an artifact of the current functional assay. The recommended diagnostic criteria for VWD include bleeding symptoms in both the patient and in family members, as well as laboratory testing indicative of low VWF levels and/or lack of function of the VWF protein.25 Reliance on the ristocetin cofactor activity assay, however, may be a source of diagnostic error in certain patients, especially those possessing SNPs that directly affect ristocetin's interactions with VWF. In addition, the presence of D1472H may affect ristocetin-induced platelet aggregation and could be a source of diagnostic error in platelet function testing. It is important to consider each patient's bleeding history, taking into account historical challenges and family history, as indiscriminate VWF testing may result in erroneous diagnosis and treatment. Alternate assays for measuring VWF function, especially those using more physiologic measures of VWF-GPIb binding, would help alleviate this problem and perhaps assist in the current problems with VWF diagnosis and classification.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the subjects, physicians, and staff involved in the ZPMCB-VWD study, ARUP Laboratories, Centers for Disease Control, Esoterix, Mayo Clinic, and Queen's University, Kingston, ON, as well as A. Lee and C. Perry, who provided research assistance.
This work was supported by the National Institutes of Health (program project grant HL081588). V.H.F. was supported by the National Hemophilia Foundation and Hemostasis and Thrombosis Research Society. R.R.M. was also supported by the National Institutes of Health (grants HL33721 and HL044612).
National Institutes of Health
Authorship
Contribution: V.H.F., J.C.G., S.L.H., and R.R.M. designed the research project; P.A.M., P.A.C., K.D.F., and B.R.B. collected data and performed experiments; R.G.H. performed the statistical analyses; V.H.F., J.C.G., S.L.H., K.D.F., T.C.A., J.A.D., W.K.H., C.L., J.M.L., M.V.R., A.D.S., and R.R.M. analyzed results; V.H.F. and R.R.M. wrote the paper; and all authors had full access to the data and edited the final paper.
Conflict-of-interest disclosure: J.C.G. is consultant to Baxter and CSL Behring. R.R.M. is consultant to GTI Diagnostics Inc, Baxter, CSL Behring, and AstraZeneca. T.C.A. is on the advisory board for CSL Behring. The remaining authors declare no competing financial interests.
Correspondence: Veronica H. Flood, Comprehensive Center for Bleeding Disorders, 8739 Watertown Plank Rd, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: vflood@mcw.edu.