Abstract
Patients with refractory anemia with ring sideroblasts and thrombocytosis (RARS-T) are difficult to treat because the cytoreductive treatment might be beneficial for the thrombocytosis component but harmful for the RARS component. As lenalidomide has shown to be efficacious in both myelodysplastic syndromes and myeloproliferative neoplasms, we have treated 2 RARS-T patients, who were transfusion dependent, with lenalidomide. We report the results of lenalidomide treatment in these patients and show that lenalidomide has clinical activity in this rare disorder. Both patients became transfusion independent, and 1 of the patients attained indeed a complete molecular remission.
Introduction
In the subgroup of myelodysplastic syndromes (MDS)/myeloproliferative neoplasms of the World Health Organization classification, refractory anemia with ring sideroblasts and thrombocytosis (RARS-T) has been proposed as a provisional entity to encompass patients who have the clinical and morphologic features of the myelodysplastic syndrome, RARS, but who also have marked thrombocytosis associated with abnormal megakaryocytes.1 In support of a myeloproliferative component to this neoplasm, the majority of cases reported as RARS-T have shown the JAK2 (V617F) mutation, or much less commonly, the myeloproliferative leukemia virus oncogene (MPL) W515K/L mutation.2-10 The clinical course of RARS-T appears to be less favorable than that of essential thrombocythemia.11 Clonality analyses and gene expression profiling suggest that RARS-T is a myeloid neoplasm with both myelodysplastic (RARS-like) and myeloproliferative (essential thrombocythemia-like) features and that it may develop from a preexisting RARS through the acquisition of somatic mutations of JAK2, MPL, or other as-yet-unknown genes.12 However, whether RARS-T is a distinct entity, or a progression of RARS resulting from an additional acquired genetic abnormality, or a form of essential thrombocythemia, remains subject of debate.13,14
Because so few cases meet the rigorous diagnostic criteria for RARS-T, there is no consensus on the optimal clinical treatment for this disorder. Lenalidomide is active against several hematologic malignancies, including lower-risk MDS with or without the 5q− cytogenetic abnormality.15-17 Clinical trials using 10 mg lenalidomide have reported cytogenetic responses of 44% and 10%, respectively, in MDS with or without 5q−.16,17 This suggests that lenalidomide can eliminate to certain degree the abnormal MDS clone. In addition, durable clinical and molecular responses have been described in myelofibrosis using the combination of lenalidomide and prednisone (7.5% partial response and 22.5% clinical improvement according to the International Working Group consensus).18
We report here 2 patients with RARS-T with a JAK2 (V617F) mutation who both became transfusion independent after treatment with lenalidomide.
Case reports
The 2 patients were treated with lenalidomide on compassionate-use basis after approval by a scientific review committee and the University Medical Center Groningen Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Case 1
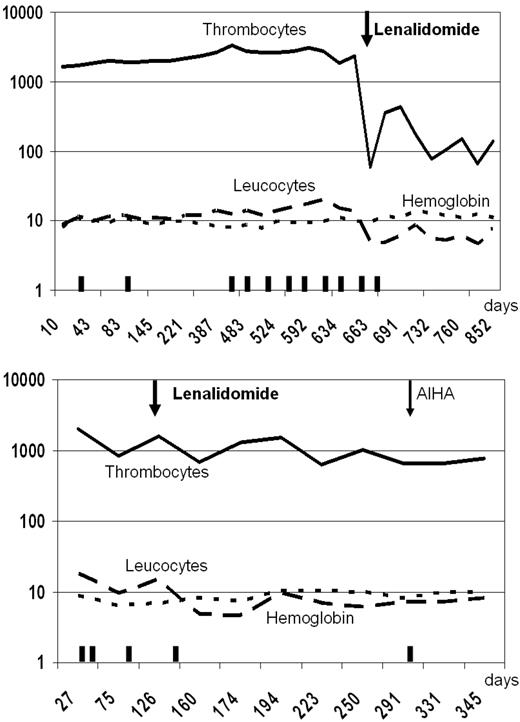
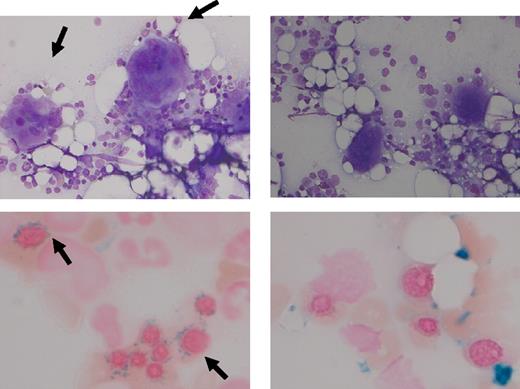
In June 2007, an 81-year-old woman with no prior history of MDS presented with anemia (mean corpuscular volume, 108 fl; hemoglobin, 4.8mM [7.9 g/dL]), leukocytes of 10.0 × 109/L, and a remarkable thrombocytosis (1677 × 109/L). The bone marrow showed approximately 60% cellularity, loose clusters of highly atypical megakaryocytes with large lobulated nuclei, and erythroid dysplasia with 86% ring sideroblasts. Metaphase cytogenetics revealed a normal karyotype. The presence of JAK2 (V617F) mutation was confirmed by quantitative polymerase chain reaction (PCR), and the amount was calculated using a calibration curve, constructed with the JAK2 (V617F)–positive HEL cell line.19 At diagnosis, a JAK2 (V617F) load of 19.8% and 17.9% was measured (duplicate). Based on these findings, RARS-T with a JAK2 (V617F) mutation was diagnosed. Treatment with erythropoietin was only temporarily successful. After almost 2 years, she presented with pulmonary hypertension resulting from chronic pulmonary embolism. Therefore, she was treated with hydroxycarbamide (hydroxyurea) 500 mg 3 times a day for 2 weeks, which successfully reduced her platelet counts. Because of her transfusion dependency (∼ 3 units of red blood cells every 4 weeks), further cytoreductive treatment was not an attractive option and she started on lenalidomide 5 mg daily. Afterward her platelet counts dropped significantly to levels approximately 100 × 109/L and her leukocytes normalized. Intriguingly, she became transfusion independent, and her hemoglobin levels almost normalized (Figure 1). After 6 months, bone marrow aspirate revealed disappearance of atypical megakaryocytes and of ring sideroblasts (Figure 2). Remarkably, the quantitative PCR for JAK2 (V617F) became negative (detection level 0.8% JAK2 (V617F) DNA). We started with the 5-mg dose because of the age of this patient and fear for pancytopenias; however, because the response was so impressive, we did not increase the dose to the standard 10-mg dose used in MDS. Currently, the patient successfully continues 5 mg of lenalidomide daily.
Peripheral blood counts and transfusions. Values of peripheral blood counts are depicted on a logarithmic scale as a function of time (in days). The y-axis represents the numbers of thrombocytes (109/L), leukocytes (109/L), and the hemoglobin level (g/dL). Top panel: Case 1. Bottom panel: Case 2.  represents the transfusion of 3 units of red blood cells.
represents the transfusion of 3 units of red blood cells.
Peripheral blood counts and transfusions. Values of peripheral blood counts are depicted on a logarithmic scale as a function of time (in days). The y-axis represents the numbers of thrombocytes (109/L), leukocytes (109/L), and the hemoglobin level (g/dL). Top panel: Case 1. Bottom panel: Case 2.  represents the transfusion of 3 units of red blood cells.
represents the transfusion of 3 units of red blood cells.
Cytomorphology. Left panel: Bone marrow aspirate of case 1 before treatment of lenalidomide. Right panel: bone marrow aspirate of case 1 after treatment of lenalidomide. The May-Grünwald-Giemsa staining (top) illustrates the disappearance of atypical megakaryocytes with large hyperlobulated nuclei; the iron staining (bottom) illustrates the disappearance of ring sideroblasts after treatment with lenalidomide. A Leica DMLB2 microscope with a Leica DC500 camera and DC TWAIN software was used to make the photograph (40× and 100× objectives).
Cytomorphology. Left panel: Bone marrow aspirate of case 1 before treatment of lenalidomide. Right panel: bone marrow aspirate of case 1 after treatment of lenalidomide. The May-Grünwald-Giemsa staining (top) illustrates the disappearance of atypical megakaryocytes with large hyperlobulated nuclei; the iron staining (bottom) illustrates the disappearance of ring sideroblasts after treatment with lenalidomide. A Leica DMLB2 microscope with a Leica DC500 camera and DC TWAIN software was used to make the photograph (40× and 100× objectives).
Case 2
In June 2007, a 60-year-old man presented with hemoglobin 4.3mM (6.9 g/dL), mean corpuscular volume 88 fl, leukocytes of 12.7 × 109/L, and thrombocytes of 1592 × 109/L. Bone marrow examination showed a cellularity of almost 100%, erythroid proliferation with myelodysplastic features and 98% ringsideroblasts, normal granulopoiesis, and a marked proliferation of atypical megakaryocytes with hyperlobulated nuclei. Metaphase cytogenetics revealed a normal karyotype. Quantitative PCR showed a JAK2 (V617F) load of 30.7% and 34.0% (duplicate). A diagnosis of RARS-T with a JAK2 (V617F) mutation was made. Treatments with pyridoxine and later anabolic steroids were not successful. In the period of 5 months before starting lenalidomide, the transfusion need was 3 units of red blood cells every 3 weeks. In March 2009, lenalidomide 10 mg daily was started, and he subsequently received only 3 units of red blood cells during the following 6 months of treatment (just after starting lenalidomide). Thrombocytes and leukocytes decreased from 1703 plus or minus 512 × 109/L and 16 plus or minus 5 × 109/L in the period before lenalidomide to 680 plus or minus 67 × 109/L and 7 plus or minus 0.5 × 109/L, respectively, after 6 months of treatment. In contrast to the first patient, the JAK2 (V617F) quantitative PCR signal did not change. In October 2009, his hemoglobin level dropped to less than 5mM (8.0 g/dL) with a marked positive direct antiglobulin test (anti-IgG, 4+) and nonspecific autoantibodies in the plasma. The autoimmune hemolytic anemia was successfully treated with prednisone while lenalidomide was continued. Autoimmune hemolytic anemia during treatment of MDS with lenalidomide has been described before.20 Currently, the patient successfully continues 10 mg lenalidomide daily.
Discussion
Because RARS-T has both myelodysplastic and myeloproliferative features, it is difficult to treat this patient group with cytoreductive therapies. Here we report clinically successful treatment of 2 RARS-T patients with a JAK2 (V617F) mutation with low-dose lenalidomide. Indeed, the JAK2 (V617F) PCR signal became undetectable in 1 patient, suggesting disappearance of the diseased clone. Unfortunately, no material was available to test this in isolated CD34+ cells. To our knowledge, this is the first report demonstrating good clinical and molecular effects of low-dose lenalidomide in RARS-T.
The favorable responses of these 2 patients to lenalidomide support the attempt of the World Health Organization classification to define RARS-T as a provisional entity. Although RARS-T is a very rare disease and its existence as disease entity is debated, the favorable clinical and molecular responses observed in the 2 described patients justify the identification of this subgroup of patients, comparable with the 5q− MDS patients. Furthermore, the disappearance of the JAK2 (V617F) in the first patient indicates that lenalidomide efficiently suppressed the JAK2 (V617F) mutated clone, either by a direct cytotoxic effect on the malignant clone or by an indirect effect, for example, via modulation of the immune system or microenvironment. Interestingly, in the presented cases, no clear evidence for either RARS or essential thrombocytosis remained detectable after lenalidomide treatment. However, it should be noted that currently no efficacy data of single-agent lenalidomide are available in essential thrombocytopenia and RARS.
The heterogeneity of the responses is remarkable. Case 1 has a complete molecular response, and case 2 has an unchanged JAK2 (V617F) signal. This might be a reflection of the heterogeneity of RARS-T. For example, recently it has been shown that approximately 25% of RARS-T patients also have a TET2 mutation, although its prognostic impact is unknown.21
In conclusion, the clinical and molecular response of RARS-T patients on lenalidomide gives good reason to identify this subgroup of patients for upfront treatment with lenalidomide.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Dr E. van den Berg for the cytogenetic analyses.
Authorship
Contribution: G.H. and J.T.M.d.W. designed research, analyzed and interpreted data, and wrote the manuscript; A.B.M. and S.R. analyzed and interpreted data; and A.A.v.d.L. and E.V. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: G. Huls, Department of Hematology, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: g.huls@int.umcg.nl; or J. T. M. de Wolf, Department of Hematology, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: j.t.m.de.wolf@int.umcg.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal