Abstract
The development of the mononuclear phagocyte system requires macrophage colony-stimulating factor (CSF-1) signaling through the CSF-1 receptor (CSF1R, CD115). We examined the effect of an antibody against CSF1R on macrophage homeostasis and function using the MacGreen transgenic mouse (csf1r-enhanced green fluorescent protein) as a reporter. The administration of a novel CSF1R blocking antibody selectively reduced the CD115+Gr-1neg monocyte precursor of resident tissue macrophages. CD115+Gr-1+ inflammatory monocytes were correspondingly increased, supporting the view that monocytes are a developmental series. Within tissue, the antibody almost completely depleted resident macrophage populations in the peritoneum, gastrointestinal tract, liver, kidney, and skin, but not in the lung or female reproductive organs. CSF1R blockade reduced the numbers of tumor-associated macrophages in syngeneic tumor models, suggesting that these cells are resident type macrophages. Conversely, it had no effect on inflammatory monocyte recruitment in models, including lipopolysaccharide-induced lung inflammation, wound healing, peritonitis, and severe acute graft-versus-host disease. Depletion of resident tissue macrophages from bone marrow transplantation recipients actually resulted in accelerated pathology and exaggerated donor T-cell activation. The data indicate that CSF1R signaling is required only for the maturation and replacement of resident-type monocytes and tissue macrophages, and is not required for monocyte production or inflammatory function.
Introduction
Mononuclear phagocytes may represent 10%-15% of the total cells in many organs of the body. Their appearance, gene expression profile, and function are very heterogeneous; the family of cells includes microglia in the brain, class II major histocompatibility complex (MHC)–expressing antigen-presenting cells (APCs) associated with most epithelia and mucosal surfaces, and bone-resorbing osteoclasts.1,2 Because of their extensive functional differences, there are few gene products that are common to all members of the mononuclear phagocyte system (MPS).3 In fact, many surface markers, such as the integrins, CD11b and CD11c, lectin-like molecules, such as sialoadhesin and macrosialin, the G-protein–coupled receptor, EMR1 (F4/80), and certain chemokine receptors (eg, CCR1, CCR2, and CX3CR1) are used rather arbitrarily to divide the MPS into putative functional subsets.3 One molecule that is expressed on the majority of cells designated as mononuclear phagocytes is the receptor for macrophage colony-stimulating factor (CSF-1), which is a type III integral membrane protein tyrosine kinase encoded by the c-fms protooncogene (csf1r).4,5 The ligand, CSF-1, controls the proliferation, differentiation, adaptation, and survival of cells of the MPS.4,5 In mice, a natural mutation of the csf1 gene, the osteopetrotic mutation (op/op), or an introduced knock out of the csf1r gene, causes a very substantial reduction in mononuclear phagocyte numbers in most tissues of the body. Even though the csf1op/op and csf1r−/− mice are viable, the importance of CSF-1-dependent macrophages in development is indicated by a failure to thrive, as well as deficiencies in development of the central nervous system, pancreas, mammary gland, and male and female reproductive function.4 The majority of the phenotypic defects seen in the csf1op/op mice, including reproductive defects and perturbations in organ development, are even more penetrant in csf1r−/− mice.6 Because of the severe pleiotropic effects of mutation of the csf1 or csf1r genes on normal development, the mutant animal phenotypes cannot address the physiologic role of CSF-1 receptor (CSF1R) signaling in an adult animal in the steady state. Such knowledge is clearly important, in light of efforts to produce selective inhibitors of CSF1R kinase activity.7,8 An alternative approach to using inhibitors, which have an intrinsic risk of cross-reactivity with related kinases, is to use antibodies against the ligand or receptor, the latter being especially relevant with the recent discovery of an alternative CSF1R ligand, interleukin-34.9
We have previously developed a mouse model in which an enhanced green fluorescent protein (EGFP) reporter gene is driven by the csf1r promoter, the MacGreen mouse line.10 In this model, the reporter gene recapitulated the expression of csf1r mRNA exclusively in myeloid cells: both mononuclear phagocytes and neutrophils. In the case of neutrophils, csf1r mRNA is not normally translated into protein, although the cells can become CSF-1–responsive and transdifferentiate into macrophages in response to CSF-1 after overnight culture.11 The transgenic reporter has been used in demonstration of CSF-1 dependence/responsiveness of APCs in lymphoid organs,12 and in direct visualization of macrophage roles in tumor progression and metastasis.13 In the current study, we used these animals as reporters to analyze the actions of a blocking monoclonal antibody (mAb) against the mouse CSF1R (CD115) on the MPS at steady state and in models of inflammation and cancer. The results indicate that the only nonredundant action of CSF-1 is to promote the maturation of blood monocytes, and suggest that existing inhibitors of CSF1R may have other activities.
Methods
MacGreen mice10 were backcrossed for 6 generations against a C57BL/6 background and were maintained in a specific pathogen-free facility. Female C57BL/6 (B6, H-2b) and B6D2F1 (H-2b/d) mice were purchased from the Animal Resource Center. Unless otherwise indicated, all mice used were female and aged 6-10 weeks, were housed in sterilized microisolator cages, and received normal chow and autoclaved water, which was acidified after transplantation. Approval for all experiments and procedures using mice was obtained from the Institutional Animal Ethics Committees of The University of Queensland and The Queensland Institute for Medical Research. M279 is a rat immunoglobulin G1 (IgG1) mAb that blocks the ability of CSF-1 to maintain proliferation of a CSF-1–dependent cell line. The characterization of this reagent and optimization of the treatment regime is provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The inflammatory and tumor models used herein, Ab reagents, and staining protocols, are all standard published methods and are described and referenced in detail in supplemental Methods and in figure legends.
Results
Anti-CSF1R Ab treatment has little effect on monocytopoeisis or blood monocyte counts
M279 is a new rat monoclonal IgG1 Ab against mouse CSF1R that, like the published antibody, AFS98,14 blocks the actions of CSF-1 on a CSF-1–dependent cell line (supplemental Figure 1). In initial experiments, we examined the effects of anti-CSF1R Ab (M279) treatment on the cellular composition of lymphoid and peripheral tissues of MacGreen mice. Utilization of the MacGreen mice facilitated the monitoring of the depletion of CSF1R+ cells via examination of the csf1r-EGFP transgene reporter expression.10,11 Based upon preliminary dose and time-course data (see supplemental Methods and supplemental Figure 2), we used a prolonged treatment regime, in which animals were treated 3× weekly for 3 weeks with 400 μg of either anti-CSF1R or control rat IgG. After prolonged Ab treatment, there was a marked decrease in the absolute number of peritoneal F4/80+ macrophages in anti-CSF1R–treated animals, compared with Ab control-treated animals (Figure 1A). However, there was no significant difference in the cellularity of bone marrow, blood, or spleen between these groups (Figure 1B). Flow cytometric analysis of EGFP+ populations including F4/80+ monocytes (not shown), Ly6G+ granulocytes, CD11b+ cells, class II+CD11chigh (referred to as conventional DCs), and CD11cdimPDCA-1+ plasmacytoid DCs (pDCs) also failed to demonstrate significant differences within the marrow. There was a trend toward a decrease in both EGFP-positive Ly6G+ and CD11b+ populations (mainly granulocytes) within the blood of anti-CSF1R–treated animals (Figure 1C).
Effect of anti-CSF1R mAb treatment on myeloid populations within lymphoid tissues. (A) Peritoneal lavage cells from anti-CSF1R and rat IgG-treated mice stained with phycoerythrin (PE)–conjugated F4/80. Bone marrow, blood, and splenocytes were counted (B) and cell populations enumerated using 3-color flow cytometry (C). (D) Flow cytometric analysis of EGFP, F4/80, and Gr-1 expression on blood mononuclear cells. In the top panel, the R3 gate contains monocytes that express 3-4-fold higher F4/80 and csf1r-EGFP, and are completely absent after antibody treatment. In the bottom panel, the R2 gate contains granulocytes, which express both Gr1 and csf1r-EGFP. These cells can also be seen in the top panel, ungated, expressed csf1r-EGFP, but not F4/80. The R3 gate contains Gr1-positive monocytes, and the R4 gate, Gr1-negative monocytes. Histograms at right compare the frequency of EGFP+F4/80high and EGFP+Gr-1neg in blood from rat IgG and anti-CSF1R treated MacGreen mice (white and black bars, respectively). Results shown are from 1 of 2 independent experiments, where n = 4-5 animals per group. A value of P < .05 was considered statistically significant (*).
Effect of anti-CSF1R mAb treatment on myeloid populations within lymphoid tissues. (A) Peritoneal lavage cells from anti-CSF1R and rat IgG-treated mice stained with phycoerythrin (PE)–conjugated F4/80. Bone marrow, blood, and splenocytes were counted (B) and cell populations enumerated using 3-color flow cytometry (C). (D) Flow cytometric analysis of EGFP, F4/80, and Gr-1 expression on blood mononuclear cells. In the top panel, the R3 gate contains monocytes that express 3-4-fold higher F4/80 and csf1r-EGFP, and are completely absent after antibody treatment. In the bottom panel, the R2 gate contains granulocytes, which express both Gr1 and csf1r-EGFP. These cells can also be seen in the top panel, ungated, expressed csf1r-EGFP, but not F4/80. The R3 gate contains Gr1-positive monocytes, and the R4 gate, Gr1-negative monocytes. Histograms at right compare the frequency of EGFP+F4/80high and EGFP+Gr-1neg in blood from rat IgG and anti-CSF1R treated MacGreen mice (white and black bars, respectively). Results shown are from 1 of 2 independent experiments, where n = 4-5 animals per group. A value of P < .05 was considered statistically significant (*).
Peripheral blood monocytes have previously been shown to be segregated into 2 functional CSF1R+ (CD115) subsets, based upon their differential expression of Gr1, an antigenic determinant shared by Ly6G and Ly6C surface molecules.15 The Gr1neg population selectively replenishes resident tissue macrophages, whereas the Gr1+ cells are short-lived and are selectively recruited to inflammatory sites.16 Thus, to further dissect the effects of anti-CSF1R treatment on blood myeloid populations, we used 3-color flow cytometry to examine the expression of Gr1 and F4/80 in association with EGFP within the blood of Ab-treated animals. Interestingly, analysis of EGFP and F4/80 expression in combination revealed that the csf1r-EGFP marker divided monocytes into 2 populations, which also differed in their expression of F4/80 antigen (Figure 1D). Furthermore, the F4/80high, EGFPhigh monocytes were also Gr1neg. After 1 week of treatment, the monocyte populations were not changed significantly (not shown), but after 3 weeks, the F4/80highEGFPhighGr1neg population was selectively and completely depleted by anti-CSF1R treatment. By contrast, the F4/80int monocytes were Gr1+, and this population was actually increased by the anti-CSF1R treatment (Figure 1D), so that the total monocyte count as a proportion of total leukocytes was maintained. The csf1r-EGFP transgene expression also detects circulating granulocytes,11 which share Gr1, but lack F4/80; these were not significantly depleted by anti-CSF1R treatment (Figure 1D). The data demonstrate that anti-CSF1R acts to prevent the maturation of the Gr+ monocytes into Gr1−/F4/80high monocytes.
The effect of anti-CSF1R treatment on resident macrophage populations
CSF-1–deficient and CSF1R-deficient mice exhibit a substantial depletion of populations of F4/80-positive macrophages in many organs of the body,4 Therefore, we next examined the effect of anti-CSF1R treatment on resident CSF1R-expressing populations in peripheral tissues of the MacGreen mice. Tissues from Ab-treated mice were harvested and examined by fluorescent microscopy for csf1r-EGFP transgene reporter expression. Anti-CSF1R treatment caused an almost complete reduction of the EGFP-positive stellate macrophage populations in liver, skin (Langerhans cells of the epidermis and the abundant population of presumed macrophages of the dermis), small intestine, stomach, colon, bladder, pancreas, testis, and kidney, compared with tissues from control rat IgG-treated animals (Figure 2A-H and supplemental Figures 3-5). Examination of hematoxylin and eosin–stained sections showed no increase in the number of pyknotic nuclei of apoptotic cells in these tissues, nor was there any overt pathology arising from the deletion of the tissue-macrophage populations (data not shown). In particular, there was no evidence of any infiltration of inflammatory monocytes or granulocytes. Both myeloid populations would likely be recruited if there was more extensive cell death and tissue damage. Both of these populations were positive for the csf1r-EGFP transgene,11 and yet, there was almost a complete removal of all transgene expression in organs such as the liver, gut, and kidney. Anti-CSF1R treatment had no effect on the brain-macrophage populations, neither those of the meninges and choroid plexus, nor the microglia of the brain parenchyma (Figure 3A-B). This was in marked contrast to the retina, in which the microglia were undetectable after anti-CSF1R treatment (Figure 3C). Three other organs, where there was minimal depletion of the csf1r-EGFP–positive macrophage populations, were the lung parenchyma and the ovary and uterus (Figure 3D-F). In the lung, the apparent overall abundance of interstitial csf1r-EGFP–positive cells was unchanged by the Ab treatment (Figure 3D). However, within the major airways, bronchoalveolar macrophages were clearly highlighted by the csf1r-EGFP transgene in control mice; most sections contained small numbers of such cells (supplemental Figure 6). By contrast, no cells clearly located within the airway space were detectable in comparable sections of lung from Ab-treated mice (not shown).
Anti-CSF1R antibody mediated depletion of CSF1R-EGFP+ tissue macrophages. MacGreen mice were treated with 400 μg of anti-CSF1R or control antibody (rat IgG) administered by intraperitoneal injection, thrice weekly for 3 weeks. Fluorescent micrographs comparing EGFP expression in 12-μm sections of liver (A), skin (B), small intestine (C), colon (D), bladder (E), pancreas (F), testis (G), and kidney (H) from anti-CSF1R or rat IgG-treated mice are shown. All images are 20× magnification.
Anti-CSF1R antibody mediated depletion of CSF1R-EGFP+ tissue macrophages. MacGreen mice were treated with 400 μg of anti-CSF1R or control antibody (rat IgG) administered by intraperitoneal injection, thrice weekly for 3 weeks. Fluorescent micrographs comparing EGFP expression in 12-μm sections of liver (A), skin (B), small intestine (C), colon (D), bladder (E), pancreas (F), testis (G), and kidney (H) from anti-CSF1R or rat IgG-treated mice are shown. All images are 20× magnification.
Tissue-specific depletion of CSF1R-EGFP+ resident tissue macrophages with anti-CSF1R antibody. MacGreen mice were treated with 400 μg of anti-CSF1R or control antibody (rat IgG) administered by IP injection, thrice weekly for 3 weeks. Shown are fluorescent micrographs of 12-μm sections of brain tissue showing parenchyma (A) and meninges (B), and of retina (C), lung (D), ovary (E), and uterus (F), from anti-CSF1R or rat IgG-treated mice. All images are 20× magnification.
Tissue-specific depletion of CSF1R-EGFP+ resident tissue macrophages with anti-CSF1R antibody. MacGreen mice were treated with 400 μg of anti-CSF1R or control antibody (rat IgG) administered by IP injection, thrice weekly for 3 weeks. Shown are fluorescent micrographs of 12-μm sections of brain tissue showing parenchyma (A) and meninges (B), and of retina (C), lung (D), ovary (E), and uterus (F), from anti-CSF1R or rat IgG-treated mice. All images are 20× magnification.
Although the anti-CSF1R treatment did not cause a global change in myeloid cellularity in lymphoid tissues (Figure 1), there was an apparent impact on certain subpopulations evident in tissue sections. In the spleen, a subset of especially bright csf1r-EGFP–positive cells surrounding lymphoid follicles (not shown) and, in the mesenteric lymph node, a population underlying the subcapsular sinus were depleted (supplemental Figure 4). Conversely, red pulp macrophages of spleen, interdigitating cells (resident DCs), medullary macrophages, and tingible body cells in the lymph nodes (supplemental Figure 4) were unaffected.
The effect of anti-CSF1R treatment on inflammatory macrophage recruitment
The lack of impact of the anti-CSF1R treatment on the inflammatory monocyte subset leads to the prediction that the treatment would not affect inflammatory processes, and might even exacerbate them. To test this prediction, we examined the effect of anti-CSF1R treatment on monocyte/macrophage recruitment in several models of inflammation. Anti-CSF1R treatment had no effect on the recruitment of monocyte/macrophages into the peritoneal cavity in response to thioglycollate broth, nor monocyte/macrophage recruitment into the lung in response to intratracheal LPS installation (Figure 4A-B). In both models, granulocyte recruitment actually appeared enhanced in the anti-CSF1R–treated animals, compared with control treated, although this was only significant in the LPS model. We also examined the effect of anti-CSF1R treatment on wound healing using a model of macrophage-dependent wound repair. Polidocanol detergent was used to strip the epithelial layer from one side of the nasal septum, while the contralateral side served as control tissue. In this model, cellular reconstitution occurs within 7 days. Neither the timing nor efficiency of tissue regeneration was affected by anti-CSF1R pretreatment (Figure 4C).
Inflammation-mediated myeloid cell recruitment and macrophage-mediated wound healing are unaffected by anti-CSF1R treatment. (A) Anti-CSF1R and rat IgG mAb-treated MacGreen mice were administered thioglycollate broth and cells within the peritoneal cavity collected by lavage 5 days later. Cells were counted, and 2-color flow cytometry was used to enumerate monocyte and granulocyte numbers. (B) BAL fluid was collected 24 and 48 hours after intratracheal instillation of LPS in anti-CSF1R and rat IgG mAb-treated MacGreen mice. Cells were counted, and 2-color flow cytometry was used to enumerate monocyte and granulocyte numbers. (C) Polidocanol detergent was used to strip the epithelial lining from one side of the nasal septum, with the contralateral side used as a control. Image shows nasal septum from anti-CSF1R and rat IgG-treated animals 9 days after polidocanol administration. Bar represents 200 μm.
Inflammation-mediated myeloid cell recruitment and macrophage-mediated wound healing are unaffected by anti-CSF1R treatment. (A) Anti-CSF1R and rat IgG mAb-treated MacGreen mice were administered thioglycollate broth and cells within the peritoneal cavity collected by lavage 5 days later. Cells were counted, and 2-color flow cytometry was used to enumerate monocyte and granulocyte numbers. (B) BAL fluid was collected 24 and 48 hours after intratracheal instillation of LPS in anti-CSF1R and rat IgG mAb-treated MacGreen mice. Cells were counted, and 2-color flow cytometry was used to enumerate monocyte and granulocyte numbers. (C) Polidocanol detergent was used to strip the epithelial lining from one side of the nasal septum, with the contralateral side used as a control. Image shows nasal septum from anti-CSF1R and rat IgG-treated animals 9 days after polidocanol administration. Bar represents 200 μm.
Having observed no change in the influx of innate macrophage populations in either inflammatory recruitment models, or in the wound-healing model, we next investigated the effects of anti-CSF1R treatment on macrophage recruitment in 2 tumor models (eg, AE5MG and Lewis Lung carcinoma), in which substantial recruitment of macrophages occurs. Tumor-associated macrophages (TAMs) are said to be alternatively activated,17 but it is not clear whether they are inflammatory or are simply resident macrophages populating a new organ (ie, the tumor). We used a subcutaneous AE5MG mesothelioma model in the MacGreen mice, in which the csf1r-EGFP marker provides a striking indication of the recruitment of macrophages into the tumor. In the mesothelioma tumor site, the csf1r-EGFP–positive cells were numerous, approximately 10%-15% of the total tumor space, and were distributed throughout the tumor. In the Lewis lung carcinoma (LLC) metastatic lesions growing in the lung, the csf1r-EGFP marker labels a similar proportion of the tumor volume (supplemental Figure 7). In each case, once the tumors were established, anti-CSF1R (or rat Ig control) treatment was initiated and its effects on the number of tumor-associated macrophages (TAMs) and tumor growth was monitored. Anti-CSF1R treatment greatly reduced the number of TAMs in the mesothelioma based on laser scanning cytometric quantitation of either GFP- or F4/80-stained sections (Figure 5A-B). In LLC, there was a similar impact, except that in some sections, there appeared to be an intense EGFP-positive inflammatory focus around the tumor periphery, which was unaffected (supplemental Figure 6). The anti-CSF1R treatment had no significant effect on tumor growth or final tumor burden in the AE5MG model (Figure 5C) or in the LLC model (not shown).
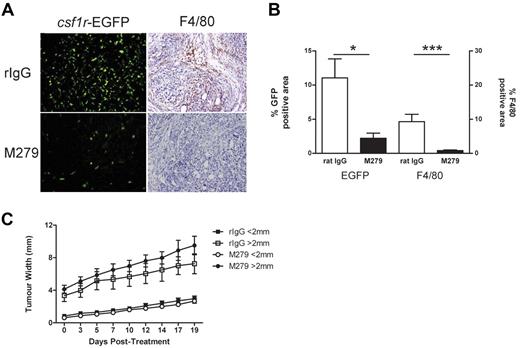
Treatment with anti-CSF1R prevented the recruitment of EGFP+ cells into tumor mass. MacGreen mice were inoculated subcutaneously with 1 × 106 mesothelioma cells and tumor nodules allowed to develop until just palpable, at which point treatment with anti-CSF1R or isotype antibody was commenced. (A) csf1r-EGFP and F4/80 expression in tumor (magnification, ×20) and (B) laser scanning quantitation of csf1r-EGFP expression and F4/80 staining within tumor. (C) Analysis of tumor growth.
Treatment with anti-CSF1R prevented the recruitment of EGFP+ cells into tumor mass. MacGreen mice were inoculated subcutaneously with 1 × 106 mesothelioma cells and tumor nodules allowed to develop until just palpable, at which point treatment with anti-CSF1R or isotype antibody was commenced. (A) csf1r-EGFP and F4/80 expression in tumor (magnification, ×20) and (B) laser scanning quantitation of csf1r-EGFP expression and F4/80 staining within tumor. (C) Analysis of tumor growth.
The effect of anti-CSF1R treatment on acute GVHD
We next wished to determine the impact of removal of the resident monocyte population and tissue macrophages in a model of immune-mediated inflammation. Graft-versus-host disease (GVHD), the major complication of bone marrow (BM) transplantation, is initiated by donor T cells, which recognize alloantigen expressed on residual host APCs. We used the well-established B6 into B6D2F1 model of acute GVHD, in which B6D2F1 recipients were first treated with anti-CSF1R or control antibody, then lethally irradiated (1100 cGy) and transplanted with allogeneic C57B6 T cell–depleted (TCD) BM with or without splenic T cells. Quite unexpectedly, the depletion of host macrophages by anti-CSF1R treatment resulted in a very significant acceleration of acute GVHD mortality (Figure 6A). Examination of GVHD target tissues, including the liver, colon, and small gut, taken 7 days after transplantation, confirmed the absence of F4/80-expressing cells in tissues from the anti-CSF1R–treated recipients (Figure 6B). Histopathologic evaluation of these tissues revealed increased gastrointestinal pathology associated with the treated transplant recipients at this time point (Figure 6C). In an effort to explain the increased pathology, we examined splenic T-cell cytokine production after transplantation. At day 7 after transplantation, interferon (IFN)γ and tumor necrosis factor (TNF) were both at significantly higher levels in the tissue culture supernatants of CD3-stimulated splenocytes purified from anti-CSF1R–treated recipients, compared with control rat IgG-treated recipients (Figure 6D). This enhanced cytokine production was attributable to an increase in the frequency of IFNγ- and TNF-expressing cells within both CD4 and CD8 T-cell compartments (Figure 6E). Although the actual number of CD4 effector cells was similar in control and anti-CSF1R–treated recipients, there was a significant decrease in FoxP3-positive regulatory T (Treg) cells in both the spleen and peripheral lymph nodes of treated recipients, resulting in a decreased CD4 Treg:effector ratio (Figure 6F). These data are consistent with the observation that anti-CSF1R does not deplete inflammatory monocytes and hence does not prevent macrophage-mediated pathology. Conversely, the CSF-1-dependent resident macrophages appear to contribute immunosuppressive activity that restricts alloreactive T-cell activation.
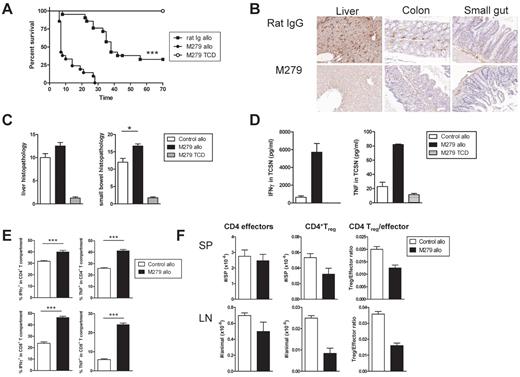
Treatment with anti-CSF1R exacerbates acute GVHD. (A) Survival by Kaplan-Meier analysis. Irradiated anti-CSF1R and rat IgG mAb-treated B6D2F1 recipients were transplanted with BM and T cells from C57Bl/6 mice as described in supplemental Methods (rat IgG allo and anti-CSF1R allo; n = 21/treatment). Irradiated anti-CSF1R–treated B6D2F1 received TCD BM and served as non-GVHD controls (n = 8). ***P < .0001, rat IgG allo vs anti-CSF1R allo. (B) F4/80 staining in liver, colon, and small gut taken from rat IgG allo and anti-CSF1R allo recipients 7 days after transplantation. (C) Liver and gut histopathology was determined by semiquantitative histology as described in supplemental Methods. (D) Unfractionated splenocytes from rat IgG allo and anti-CSF1R allo recipients 7 days after transplantation were cultured with CD3 and IFNγ and TNF determined in 24-hour tissue-culture supernatant or (E) cultured with CD3 and brefeldin and IFNγ and TNF measured by intracellular cytokine staining. (F) CD4+FoxP3− T effectors and CD4+FoxP3+ Treg were enumerated in spleens and lymph nodes from rat IgG allo and anti-CSF1R allo recipients 7 days after transplantation. In all histograms, the white bar represents rat IgG allo recipients; the black bar, anti-CSF1R allo recipients; and the hatched bar, anti-CSF1R TCD recipients.
Treatment with anti-CSF1R exacerbates acute GVHD. (A) Survival by Kaplan-Meier analysis. Irradiated anti-CSF1R and rat IgG mAb-treated B6D2F1 recipients were transplanted with BM and T cells from C57Bl/6 mice as described in supplemental Methods (rat IgG allo and anti-CSF1R allo; n = 21/treatment). Irradiated anti-CSF1R–treated B6D2F1 received TCD BM and served as non-GVHD controls (n = 8). ***P < .0001, rat IgG allo vs anti-CSF1R allo. (B) F4/80 staining in liver, colon, and small gut taken from rat IgG allo and anti-CSF1R allo recipients 7 days after transplantation. (C) Liver and gut histopathology was determined by semiquantitative histology as described in supplemental Methods. (D) Unfractionated splenocytes from rat IgG allo and anti-CSF1R allo recipients 7 days after transplantation were cultured with CD3 and IFNγ and TNF determined in 24-hour tissue-culture supernatant or (E) cultured with CD3 and brefeldin and IFNγ and TNF measured by intracellular cytokine staining. (F) CD4+FoxP3− T effectors and CD4+FoxP3+ Treg were enumerated in spleens and lymph nodes from rat IgG allo and anti-CSF1R allo recipients 7 days after transplantation. In all histograms, the white bar represents rat IgG allo recipients; the black bar, anti-CSF1R allo recipients; and the hatched bar, anti-CSF1R TCD recipients.
Discussion
The data presented herein unify and extend previous findings concerning the role of CSF1R signaling in monocyte-macrophage development. We have used prolonged treatment with anti-CSF1R Ab to deplete the large majority of tissue macrophage populations. The M279 anti-CSF1R mAb is a rat IgG1, a subclass that does not fix complement. By contrast to other approaches, such as the various transgenic lines expressing the diptheria toxin receptor in myeloid cells,18 the so-called MAFIA mice,19 or the use of toxic liposomes,20 the Ab did not induce an immediate loss of tissue macrophages or blood monocytes, nor was there any evidence of local inflammation. It clearly does not deplete all of the cells that bear the CSF1R on their surface, because it had no effect on the inflammatory monocytes or their marrow precursors. Because one function of apoptosis is to attract monocytes to clear the dying cells,21 and because the antibody did not actually prevent the recruitment of inflammatory monocytes in any of the models we have studied, acute apoptosis is an unlikely explanation for the observed complete depletion of csf1r-EGFP–positive cells from most organs.
Instead, we suggest that the Ab depletes the subset of monocytes that has been shown, by others, to be committed to a resident tissue macrophage fate16 and thereby prevents replacement of tissue macrophages as they turn over normally. The depletion of the resident monocytes was balanced by an increase in the inflammatory monocytes, so that total monocyte numbers were unaffected. The proposed model is consistent with evidence of a precursor relationship between the immature inflammatory monocyte and mature resident monocytes in vivo.15,22 The time course of the response to anti-CSF1R is also consistent with the proposed mechanism. We did not see significant monocyte depletion after 1 week of treatment. Tacke et al22 used a labeled phagocytic tracer in mice to demonstrate that Gr1high monocytes convert to Gr1neg monocytes over a period of 5-7 days, and labeled Gr1neg monocytes disappear from the circulation with a half-life of approximately 7 days. The ability of CSF-1 to promote monocyte maturation and acquisition of the resident phenotype in vitro is well documented.15 Bogunovic et al have recently demonstrated that adoptively transferred csf1r−/− fetal liver cells into irradiated recipients gives rise to Gr1high but not Gr1lo blood monocytes.23 So, the actions of anti-CSF1R indicate that CSF-1, which is present at biologically active concentrations in the circulation, is the missing factor22 that promotes the maturation of the Gr1high to the Gr1neg monocyte. The so-called resident monocyte is characterized by a CD115+Gr-1neg phenotype and high expression of CX3CR1 (and of a CX3CR1-EGFP reporter transgene)16 Herein, we add 2 additional phenotypic characteristics to these cells in that they have 3-4-fold higher expression of both the F4/80 macrophage-specific antigen and the csf1r-EGFP transgene (Figure 1). The mechanism underlying increased expression of the csf1r-EGFP reporter with monocyte maturation is unclear; it presumably reflects increased expression of macrophage-specific or -inducible transcription factors that act upon the csf1r promoter and intragenic enhancer.10 However, we have confirmed in other studies that the Gr1high, csf1r-EGFPlow monocytes are selectively recruited into the peritoneum in response to foreign bodies,24 so it provides a convenient additional marker for studies of monocyte-macrophage function during inflammation.
We cannot eliminate the possibility that anti-CSF1R accelerates the turnover of tissue macrophages, in addition to its impact on their monocytic precursors. However, the time frame for the depletion of tissue macrophages is consistent with many studies on the turnover of tissue macrophages, in which alveolar macrophages turned over on average every 27 days, and Kupffer cells of the liver every 21 days.25,26 Monocyte and tissue macrophage numbers increase rapidly (within 4 days) after intravenous CSF-1 injection,27 which we would now suggest is due to monocyte maturation rather than increased production. We noted complete depletion of the alveolar macrophages in the airways in sections of lung after Ab administration, supporting the view that they are CSF-1 dependent.28 This is not incompatible with recent evidence that they are not replaced directly from monocytes29 ; it may mean that they depend more on local CSF-1 production and are produced locally. By contrast, the interstitial csf1r-EGFP–positive cells of the lung remained unaffected by the Ab treatment (Figure 3D). These cells also appear not to be derived directly from monocytes in the steady state, and, in contrast to alveolar macrophages, presumably do not require CSF-1 signaling.29
The csf1r-EGFP transgene is expressed in all myeloid cells, including all currently described subsets of DCs.10-12 The anti-CSF1R Ab treatment depleted most EGFP-positive cells in the majority of organs. Among the most dramatic effects was the almost complete absence of EGFP-positive cells from the wall of the GI tract after anti-CSF1R treatment (Figure 2C-D and supplemental Figure 5). Lamina propria macrophages are the largest macrophage population in the body.1,2 There is an almost complete overlap between the macrophage-specific F4/80 antigen and CD11c in this location, as well as complete overlap of csf1r-EGFP transgene and F4/80.30 Several groups have recognized that the lamina propria mononuclear phagocytes can be divided into those that can stimulate and those that repress T cell–mediated immune responses.31,32 Based on the absence of EGFP and F4/80 expression within the gut (Figures 2C-D,6B and supplemental Figure 5), anti-CSF1R Ab treatment removes both populations. Notably, the lamina propria mononuclear phagocytes are apparently derived from circulating monocytes in adoptive transfer studies33 and are absent in csf1op/op and csf1r−/− mice.4
The macrophage populations of the dermis and epidermis, including Langerhans cells, were also completely depleted by the anti-CSF1R treatment (Figure 2B), in keeping with their absence from csf1r−/− mice.4 In inflamed skin, these cells have been shown to be replenished from circulating Gr-1+ monocytes: the inflammatory subset.34 Our study suggests that in the normal steady state, when they perhaps turn over much more slowly, skin macrophage populations might actually be replenished from the resident monocyte population. However, Chorro et al35 have suggested that Langerhans cells are established from a wave of migratory progenitors shortly after birth and thereafter are replenished by local proliferation. So, the alternative view is that the local progenitor is CSF-1 dependent. In any case, the turnover of these cells would appear to be relatively rapid in the steady state, and the Ab treatment provides a novel model for the study of myeloid APCs of the skin.
Similarly, in the brain, microglia are believed to be replenished from the circulating monocyte pool only under inflammatory conditions, and in this circumstance, the Gr-1+ inflammatory monocytes appear to be the precursors.36 We saw no effect of the anti-CSF1R Ab on brain microglia, but the microglia of the retina were completely depleted by the Ab (Figure 3C). This difference may be a function of the blood-brain barrier; however, it could also be an issue of the rate of turnover and replacement, because only 23% of brain microglia are of donor origin in BM chimeras 6 months after transplantation.37 The majority of the macrophage populations of the spleen and lymph nodes were unaffected by the Ab treatment, and by extension, are probably not derived from a CSF-1–dependent resident monocyte or controlled by local CSF-1 production. The likely explanation is that these sites are seeded by progenitors divergent from more mature monocytes that also proliferate locally.38,39 The exception is the marginal zone macrophages of the spleen, and the subcapsular sinus macrophages of the lymph node, which seem to express the csf1r-EGFP transgene at high levels and which were depleted in Ab-treated animals. Marginal-zone macrophages have been shown previously to be CSF-1 dependent.40
The simplest explanation for the failure of the Ab treatment to alter the course of inflammation in any of the models we have tested is that it does not remove the inflammatory monocyte subset. Importantly, because CSF-1 is elevated in the circulation in many inflammatory states,4,5 the data demonstrate that CSF-1 is not required for increased monocytopoiesis in response to inflammatory stimuli. The failure of anti-CSF1R to inhibit thioglycollate-elicited macrophage recruitment to the peritoneum contrasted with a report that an orally available CSF-1 kinase inhibitor, GW2580, inhibited this response.7 Subsequently, GW2580 was reported to inhibit many inflammatory responses in rat models.8 But, in complete contrast to the anti-CSF1R Ab in our study, GW2580 actually caused a substantial rise in blood monocyte count. GW2580 is an effective inhibitor of the related flt3 and c-kit tyrosine kinases;41 thus, the contrast with the actions of anti-CSF1R suggests that the inhibitor does not act specifically via CSF1R. Another inhibitor described more recently, JNJ-28312141,42 also had a different effect to the anti-CSF1R Ab, causing acute depletion of tissue macrophages detected with F4/80 within 4 days. In this case, one might be concerned with the marker used, because F4/80 is itself CSF-1 dependent.27 JNJ-28312141 was proposed as a cancer therapeutic, with the proposed mechanism based upon TAM depletion.42 Again, we suspect that the inhibitor could act on targets other than CSF1R, but it might also be the case that it penetrates more effectively to tissue macrophages—and they are, in fact, CSF-1 dependent for survival.
In the epithelial damage wound healing model (Figure 4C), we saw no impact of the Ab treatment on macrophage recruitment or repair. We might also consider the ovary and uterus, where anti-CSF1R had no impact on the abundant csf1r-EGFP–positive populations (Figure 3), as forms of tissue repair, because there is considerable tissue turnover through the estrous cycle. By contrast, others have found that anti-CSF1R Ab AFS98 can impair recruitment and local proliferation in wound healing/repair, for example, after injury in the kidney and in muscle.43,44 In these cases, and in the tumors, macrophage proliferation in response to CSF-1 produced locally could be involved.43 There is a substantial body of literature on the cellular phenotype of TAMs, much of it focused on the existence of an alternative activation state referred to as the M2 type.17 The csf1r-EGFP transgene provides a striking view of the extent of macrophage infiltration in tumors (Figure 5 and supplemental Figure 6). However, the substantive depletion of TAMs by anti-CSF1R treatment suggests that they could primarily be resident type cells. In the 2 tumor models we studied, and with the timing and conditions employed, there was no impact of substantial macrophage depletion on tumor growth. According to our model, anti-CSF1R acts to deplete the macrophages already present in the established tumors, and any impact on the growth of the rapidly growing established mesothelioma tumor we studied would likely be delayed beyond the 3-week time course examined. In the LLC metastatic lung model, although the Ab-depleted csf1r-EGFP macrophages from the tumor mass (supplemental Figure 6), it did not prevent their accumulation around the invasion front, and it did not deplete lung-parenchymal macrophages. So, the LLC tumors were not deprived entirely of potential trophic actions of macrophages. The abundant evidence that macrophages are required for tumor growth in human and experimental tumors13,17 suggests that such depletion could be of benefit to cancer patients, and CSF1R remains a candidate anticancer drug target.
Others have also concluded that the inflammatory monocyte subset contains the major effectors in experimental autoimmune encephalitis45 and lupus nephritis.46 Interestingly, although anti-CSF1R does not deplete the total monocyte pool in these pathologies, CSF-1 treatment can greatly expand it,27 and it is the inflammatory monocyte subset that is increased.46 Thus, the production of inflammatory monocytes in the steady state is responsive to, but not dependent upon, circulating CSF-1 signaling through the CSF-1 receptor. The model in which there was a striking effect of anti-CSF1R was GVHD. The most important finding in terms of the current study is that the Ab did not prevent pathology in an immune-mediated inflammatory model, reinforcing the fact that CSF-1 signaling does not have a nonredundant function in inflammation. The unexpected finding was that the Ab treatment actually exacerbated the disease. In the GVHD model, inflammation is driven by activation of host APCs, stimulation of alloreactive donor T cell–derived T helper 1 cytokines and the release of TLR ligands from the irradiated GI tract.47 Anti-CSF1R pretreatment of BM graft recipients actually increased T-cell activation and proinflammatory cytokine production, decreased numbers of regulatory T cells (Figure 6), and generated a comparative increase in the inflammatory monocyte subset (Figure 1D). Together, these changes provide a possible explanation of the accelerated pathology. We and others have shown that CSF-1 is a mediator of the immunosuppression associated with cancer-bearing or chronic infection.48,49 Dissection of the precise mechanism of action of anti-CSF1R on allogeneic T-cell stimulation is beyond the scope of this study; it likely involves the removal of tolerogenic APCs in the wall of the gut,31 as well as CSF-1–dependent myeloid suppressor cells49 that would constrain the development of donor-derived alloreactive T cells. The treatment is equivalent in some respects to the knockout of the CSF-1–responsive F4/80 gene, which also worsens autoimmune-mediated pathology and reduces production of regulatory T cells.50 From a therapeutic viewpoint, one might consider using CSF-1 to ameliorate disease, although such treatment would also expand the inflammatory monocyte pool.45,46 Conversely, one might consider anti-CSF1R as an approach to restoring immune responses in immunosuppressed patients.
The final question is what is the exact function of CSF1R signaling in resident monocytes. One of the known actions of CSF-1 is to maintain macrophage survival. Lagasse and Weissmann reported that transgenic expression of the antiapoptotic Bcl-2 gene in myeloid cells was sufficient to counter, in part, the op/op mutation, permitting replenishment of many tissue-macrophage populations.51 As noted above, the resident monocytes are comparatively long lived in the circulation, whereas their precursors, the inflammatory monocytes, are short lived.22 Logically, the large majority of monocytes that enter the circulation must exit or die, and only a small subset matures to become resident type cells. In keeping with this view, anti-CSF1R did not escalate circulating monocyte numbers and did not cause any increase in inflammatory monocytes in tissues, even though it did not prevent their infiltration in response to a stimulus. CSF-1 could simply act to maintain the survival and increase the half-life of immature monocytes, thereby exerting a permissive, rather than an instructive, role in monocyte maturation. Whatever the mechanism, our data suggest that the ability of systemic treatment with CSF-1 to increase monocyte numbers and tissue mononuclear-phagocyte populations27,52 is a consequence of enhanced differentiation of the short-lived Gr-1+ monocytes, rather than increased production of monocytes from the BM. Indeed, Sudo et al14 have suggested that stem cell factor, acting through c-kit, is more important than CSF-1 for the proliferation of BM monocyte progenitors.
The analysis of animals treated with anti-CSF1R Abs strongly supports the emerging view of functional monocyte heterogeneity and provides a unique model in which tissue macrophage populations can be selectively depleted. At least in the steady state, there is remarkably little impact on the health of the animals, and this may represent a novel approach to modulate immunologic responses in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to Erica A. Dearstyne for her excellent technical work on the laser-scanning cytometry of tumor macrophages. Jayne Schaubhut and Courtney Clark (CSF-1 ELISAs), and Bryan Kennedy (DRM assay) also provided technical assistance.
Authorship
Contribution: K.P.A.M. and J.S.P. designed and performed studies and helped write the manuscript; S.C., E.S., S.O., N.C.R., R.K., and A.R.P. performed experiments; A.C. performed all blinded histologic analysis; B.W. supported and supervised studies on inflammatory models; D.B., J.S., R.J.P., D.P.C., are L.B. are current or former Amgen employees involved in the production and characterization of the M279 Ab, initiation of the collaboration, and design of the studies; and G.R.H. and D.A.H. designed studies and helped write the manuscript.
Conflict-of-interest disclosure: D.B., J.S., R.J.P., and L.B. are employed by a company with a commercial interest in reagents that are relevant to this study. The remaining authors declare no competing financial interests.
Correspondence: David Hume, The Roslin Institute, University of Edinburgh, Roslin EH25 9PS, Scotland, United Kingdom; e-mail: David.Hume@roslin.ed.ac.uk.
References
Author notes
K.P.A.M. and J.S.P. contributed equally to this article.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal