Abstract
The nuclear factor of activated T cells (NFAT) family of transcription factors functions as integrators of multiple signaling pathways by binding to chromatin in combination with other transcription factors and coactivators to regulate genes central for cell growth and survival in hematopoietic cells. Recent experimental evidence has implicated the calcineurin/NFAT signaling pathway in the pathogenesis of various malignancies, including diffuse large B-cell lymphoma (DLBCL). However, the molecular mechanism(s) underlying NFATc1 regulation of genes controlling lymphoma cell growth and survival is still unclear. In this study, we demonstrate that the transcription factor NFATc1 regulates gene expression in DLBCL cells through a chromatin remodeling mechanism that involves recruitment of the SWItch/Sucrose NonFermentable chromatin remodeling complex ATPase enzyme SMARCA4 (also known as Brahma-related gene 1) to NFATc1 targeted gene promoters. The NFATc1/Brahma-related gene 1 complex induces promoter DNase I hypersensitive sites and recruits other transcription factors to the active chromatin site to regulate gene transcription. Targeting NFATc1 with specific small hairpin RNA inhibits DNase I hypersensitive site formation and down-regulates target gene expression. Our data support a novel epigenetic control mechanism for the transcriptional regulation of growth and survival genes by NFATc1 in the pathophysiology of DLBCL and suggests that targeting NFATc1 could potentially have therapeutic value.
Introduction
Diffuse large B-cell lymphoma (DLBCL), an aggressive form of non-Hodgkin B-cell lymphoma (NHL-B), is the most common subtype of aggressive NHL-B, accounting for more than 30% of all NHL-B cases.1-3 The pathophysiology of DLBCL appears to depend on several growth and survival signaling pathways,4-6 including nuclear factor of activated T cells (NFAT),5,7,8 a well-known family of transcription factors that play important roles in regulation of the immune system, best known for their critical roles in T-cell activation and cytokine transcriptional regulation.9,10 Our understanding of the molecular mechanism(s) controlling B-cell lymphoma cell growth and survival mediated through the NFAT pathway is still incomplete however and requires further elucidation.
Activation of the NFAT signaling pathway has been implicated recently in both hematologic and solid tumors in neoplastic development.11,12 NFATc2/NFAT5 expression and transcriptional activation are induced downstream of integrin signaling, promoting carcinoma cell migration and invasion in a mouse carcinoma model.13 Constitutive activation of NFATc1 has been found in approximately 70% of pancreatic carcinomas, and blocking NFATc1 activation with cyclosporin A inhibited both cell growth and survival in a pancreatic tumor cell line.14 A recent immunohistologic study showed nuclear expression of NFATc1 in some cases of DLBCL,8 but a more recent study indicated that NFATc1 is overexpressed in a subset of DLBCL due to genomic amplification.7 This is probably not surprising, because NFATc1 and NFATc2 have long been described as also functional in the B-cell lineage, but their roles and importance in normal and neoplastic B-cell biology have not been substantively pursued or elucidated. The significance of these findings poses important translational research questions regarding the molecular and cell biology of NFAT proteins and their key roles in controlling cell proliferation, survival, and other biologic functions in neoplastic as well as normal B-lymphoid cells. It has become increasingly apparent that, in addition to its role in T-lymphocyte activation, NFAT is also involved in critical aspects of malignant cell transformation and tumorigenic processes.15,16
Although we have shown previously that NFAT family member NFATc1 is constitutively activated and can maintain cell growth and survival in DLBCL cell lines and primary cells,5 the molecular mechanism(s) underlying NFATc1 regulation of cell growth and survival in DLBCL is still unclear. Studies in other lymphoid and hematopoietic cell types (eg, T cells, mast cells) by Cockerill17 and Goldfeld's group18 have indicated that, in closely linked genes such as GM-CSF and IL3, NFAT plays a major role in chromatin remodeling, forming DNA hypersensitive sites (DHSs) in enhancer/promoters, functioning to disrupt nucleosomes to increase DNA accessibility to other transcription factors and cofactors while synergizing transcription factor/cofactor binding to targeted promoters/enhancers. We have previously detected specific interactions between NFAT and nuclear factor-κB (NF-κB), particularly in the DLBCL CD40L (CD40 ligand, also known as CD154) and BLyS (also known as BAFF) gene promoters.5,19 We have hypothesized that types of chromatin remodeling and other enhancer activation mechanisms similar to those reported in the T-cell GM-CSF gene, are also active in DLBCL NFAT-targeted growth and survival genes.
Changes in chromatin structure are catalyzed by ATP-dependent chromatin remodeling enzymes through 1 of 2 mutually exclusive subunits, brahma (Brm) and brahma-related gene-1 (Brg-1). While both Brg-1 and Brm can function as the central ATPase in the SWItch/sucrose nonfermentable (SWI/SNF) chromatin remodeling complex, each defines a discrete complex with unique biochemical activity.20 Because of its central function in epigenetic chromatin-remodeling mechanisms, dysregulation of SWI/SNF and its ATPase can lead to tumor development and growth.21 However, the impact of the chromatin remodeling mechanism in the biology of B-cell lymphomas is still unclear and unproven.
In this study, we demonstrate that transcription factor NFATc1 regulates growth and survival genes in DLBCL cells through a chromatin remodeling mechanism that involves recruitment of the SWI/SNF chromatin remodeling complex. In representative aggressive B-cell lymphoma cell lines, we found that, besides the critical TNF ligands CD40L and BlyS,5,19 the gene encoding the c-myc oncoprotein is also a target of NFATc1. Using the transcriptional regulation of c-myc by NFATc1 as a biologic model system, we discovered that the SWI/SNF ATPase Brg-1 was in fact involved in the NFATc1-mediated chromatin-remodeling mechanism by inducing DHS in DLBCL cells. Our data indicate a novel epigenetic chromatin remodeling mechanism for the transcription factor NFATc1 in the pathophysiology of aggressive lymphoma B cells and suggest that targeting NFATc1 could have therapeutic value.
Methods
Cells and reagents
Human DLBCL cell lines (MS, DS, DB, JM [McA], FN, EJ, HF, HB, MZ, LR, CJ, LP, and PL) were established from tissue biopsy or effusion specimens from patients as described elsewhere.22 The SUDHL-4 and OCI-LY10 DLBCL cell lines were obtained from Dr Michael Rosenblum (The University of Texas M. D. Anderson Cancer Center, Houston, TX). The Ramos and BJAB cell lines were obtained from ATCC. All cell lines, except for Ramos, EJ, CJ, and HB, were negative for c-myc t(8;14) translocation. This study was conducted in accordance with the Helsinki protocol and approved by the Institutional Review Board of The University of Texas M. D. Anderson Cancer Center. Informed consent was obtained from all patients whose tumor samples were used. The cells were cultured in RPMI medium (Invitrogen) containing 15% fetal calf serum (Hyclone).
Antibodies and plasmids
The following primary antibodies were used: polyclonal Brg-1 and Oct-1 and monoclonal NFATc1, NFATc2, c-myc, brm (Santa Cruz Biotechnology), and polyclonal p65 and c-rel and monoclonal STAT3 (Millipore). The SureSilencing small hairpin (sh) RNA green fluorescent protein (GFP)–based plasmids for NFATc1 and the negative control (set of 4 plasmids) were purchased from SuperArray Biosciences. The c-myc reporter constructs (Del-1 and Del-6) were obtained from Addgene.23,24 The Del-6 mutant was generated from the Del-6-wt reporter construct using the QuickChange site-directed mutagenesis Kit (Stratagene). Mutagenesis primers were 5′-CTCAGAGGCTTGGCGGGCCCAAGAACGGAGGGAG-3′ and its complementary strand. The NFATc1 eukaryotic expression vector construct pSH160c (kindly provided by Dr Gerald R. Crabtree, Department of Pathology, Stanford University, Palo Alto, CA) contains the human NFATc1 cDNA (nucletotide 243 to 2751) tagged at the N-terminus with a Flag epitope linked in-frame with the second codon.25 The dominant-negative–NFAT expression vector was a gift from Dr Chi-Wing Chow (Department of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, NY).26 The caNFATc1 mutant (pMSCV-caNFATc1) and control retrovirus plasmids were provided by Dr Neil Clipstone (Northwestern University, Chicago, IL).16
Tissue microarray analysis and immunohistochemistry
DLBCL tissue microarrays, containing 100 cases of LBCL (single core per case; LY1001), were purchased from US Biomax. The tissue microarray analysis (TMA) slides were dewaxed at 55°C for 20 minutes followed by three 5-minute washes with xylene. Immunostaining was performed using 5-μm-thick, formalin-fixed, paraffin-embedded tissue sections, epitope retrieval with Diva deblocking buffer, a deblocking chamber (Biocare Medical), and the Mach 3 system (Biocare Medical). Staining was performed using the Autostainer Plus (DakoCytomation). The washing buffer used was 0.05M Tris-buffered saline supplemented with 0.05% Tween. 3,3′-diaminobenzidine tetrahydrochloride was used as the chromogen (Liquid DAB+ Substrate Chromogen System; DakoCytomation), and all tissue sections were counterstained with hematoxylin. The evaluation of NFATc1 and c-myc staining were semiquantitatively scored using the Applied Imaging Ariol automated analysis system (Genetix). Microarray positivity for NFATc1 and c-myc was defined as immunostaining in greater than 30% of the total cells (30% cutoff). Nuclear and cytoplasmic staining was evaluated by 3 individuals (L.V.P., A.T.T., and R.J.F.). Tissue sections from normal tonsil were used as controls for NFATc1 immunostaining. TMA photomicrographs were captured using an Olympus BX41 dual-head light microscope equipped with an Olympus Q-Color 5 digital camera (Olympus America), with a 20× plan-apochromat objective. Digital images were obtained and adjusted using Adobe Photoshop CS3 (Adobe Systems).
Analysis of NFAT DNA-binding activity
Electromobility gel-shift assays were performed according to procedures described previously.6 The DNA-binding activity of NFAT and other factors to the NFAT consensus site was analyzed by an enzyme-linked immunosorbent assay (ELISA)–based assay according to the manufacturer's instructions (TransAM NFAT Family Transcription Factor Assay Kit; Active Motif). Briefly, nuclear extracts were added to the wells of a 96-well plate that contained the immobilized oligonucleotide carrying an NFAT consensus site. Proteins bound to this immobilized oligonucleotide were detected by incubating with a primary antibody that recognizes active NFATc1 and other transcription factors, followed by horseradish peroxidase–conjugated secondary antibody, and were quantified by spectrophotometry at 450 nm with a reference wavelength of 650 nm.
Coimmunoprecipitation procedures
Antibodies were crosslinked to Dynabeads Protein A (Dynal Biotech) according to the manufacturer's directions. Cell lysates were precleared with IgG Dynabeads Protein A for 30 minutes at 4°C before incubation with antibody–linked Dynabeads overnight at 4°C. The immunoprecipitated Dynabeads complexes were washed 5× with immunoprecipitation buffer (10mM Tris-HCl [pH 7.8], 1mM EDTA [ethylenediaminetetraacetic acid], 150mM NaCl, 1mM NaF, 0.5% Nonidet P-40, 0.5% glucopyranoside, 1 μg/mL aprotinin, and 0.5mM phenylmethylsulfonyl fluoride). Proteins were eluted by boiling in protein-loading buffer and then processed for Western blot analysis.
ChIP assays
Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP assay kit and protocol provided by Millipore. Cells were crosslinked with 1% formaldehyde in medium for 10 minutes at 37°C, washed with cold 1× phosphate-buffered saline (PBS), resuspended in cell sodium dodecyl sulfate lysis buffer (provided with kit) for 10 minutes on ice, and sonicated at 10-second intervals 3× with a sonicator. Samples were subjected to centrifugation for 10 minutes at 16 000g (13 000 rpm) at 4°C, and the supernatants were diluted with ChIP dilution buffer. To reduce nonspecific background, samples were precleared with protein A Dynabeads with normal immunoglobulin G (IgG) for 30 minutes at 4°C with agitation. Primary antibodies were added to the samples, which were incubated overnight at 4°C. The protein A-antibody–DNA complexes were washed (8×) and eluted according to the manufacturer's protocol and then reverse crosslinked by heating at 65°C for 4 hours. DNA fragments were treated with proteinase K and purified using the QIAGEN PCR purification kit. Purified DNA from immunoprecipitation studies and DNA inputs were used for quantitative real-time (rt) polymerase chain reaction (Q-PCR). Data were analyzed using the SuperArray ChIP-QPCR Data Analysis Template (SABiosciences).
Quantification of DNase I sensitivity
DNase I sensitivity analysis was performed according to the protocol of McArthur et al.27 DLBCL cells were harvested and resuspended in ice-cold lysis buffer (100mM KCL, 50mM Tris-CL [pH 7.9], 50% [vol/vol] glycerol, 200mM β-mercaptoethanol, and 5mM MgCl2) and incubated for 10 minutes. Nuclei were recovered from the lysed cells by subjecting the suspension to centrifugation at 13 000g for 15 minutes at 4°C and resuspending the cells in Buffer A (50mM Tris-Cl [pH 7.9], 3mM MgCl2, 0.2mM phenylmethylsulfonyl fluoride, 100mM NaCl, and 1mM dithiothreitol). Nuclei were digested with the indicated amount of DNase I for 3 minutes at room temperature in Buffer A. The samples were then treated with proteinase K and RNase A, and the DNA was recovered using the QIAGEN PCR purification kit. DNase-treated DNA was subjected to Q-PCR.
RNA isolation and rt PCR
Total RNA isolation was performed using Trizol LS Reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription of RNA was carried out with cDNA archive kit (Applied Biosystems). Synthesized cDNA was subjected to Q-PCR for the detection of related genes transcripts (C-MYC, NFATc1, and 18S). In brief, 2.5 μL of cDNA was placed in a 25-μL reaction volume containing 12.5 μL of TaqMan Universal PCR Master Mix, No AmpErase UNG, 8.75 μL of water and 1.25 μL of primers and probe sets. The primers and probes were purchased from Applied Biosystems (C-MYC: HS9999903; NFATc1: HS00542678; 18S: HS03003631). Primers and Probes were designed to span exon–exon boundaries. Amplification was performed in ABI Fast 7500 Real Time PCR system (Applied Biosciences) using the cycling program: 95°C for 10 minutes; 40 cycles of 95°C for 15 seconds, 60°C for 60 seconds. All samples were analyzed in triplicate. DNA contamination was evaluated by performing PCR on the nonreverse transcribed control of each sample. The relative expression levels of the genes of interest were normalized to endogenous reference 18S and relative to a control sample as a calibrator using the formula: 2−ΔΔCT. The Threshold Cycle reflects the cycle number at which the fluorescence generated within a reaction crosses the threshold.
Q-PCR for ChIP and DNase1 assays
DNA samples from ChIP assays or DNase I sensitivity assays and primers were added to the additional rt PCR reagents (RT2 SYBR Green/ROX qPCR Master Mix) in a 25-μL reaction volume and subjected to the following cycles: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds), by the ABI7900 HT (Applied Biosystems). No-template reactions (primers only) were used for normalization. Each sample was amplified in triplicate. Three independent experiments were performed. Averages and SDs were calculated on the basis of relative copy numbers from each experiment. Q-PCR primers were purchased from SABiosciences: NFAT2 binding site in the c-myc gene promoter (TF-BS position:128818043); NFAT2 binding site in the CD40L gene promoter (TF-BS position:13551054); NFAT2 binding site in the BlyS gene promoter (TF-BS position) and Nf-M gene promoter (a known DNase 1-resistant locus); the primers synthesized were Nf-Mf, 5′-GCT GGG TGA TGC TTA CGA CC-3′ and Nf-Mr, 5′-GCG GCA TTT GAA CCA CTC TT-3′ (988-1438; GI: 53 357).
Transient transfection of lymphoma cells
DLBCL-MS cells were transiently transfected with 2-10 μg of plasmids using the Neon Transfection System (Invitrogen). A set of 4 shRNA plasmids for NFATc1 was tested, and the optimal gene knockdown shRNA plasmid was selected for further studies. The transfection efficiency ranges from 70%-80% with 75% cell viability for MS cells, from 50%-60% with 75% viability for SUDHL-4 cells, and from 50%-60% with 70% viability for OCI-LY10 cells. Luciferase and beta-galactosidase assays were performed according to the manufacturer's instructions (Promega).
Production of recombinant retroviruses and infection of lymphoma cells
Recombinant retroviruses were produced by cotransfecting either the pMSCV-GFP or pMSCV-caNFATc1 proviral vectors together with pVSV-G (Clontech), encoding the vesicular stomatitis virus-glycoprotein, into the GP293 pantrophic packaging cell line (Clontech) using FuGENE6 (Roche). Medium was replaced after 24 hours, and viral supernatants were harvested 2 days after transfection and stored at −80°C. For infections, lymphoma cells were incubated in viral supernatant containing 8 μg/mL polybrene (Sigma-Aldrich), and were centrifuged at 2000 rpm for 1.5 hours at room temperature. Cells were expanded in growth medium for subsequent analysis and typically used within 2-4 days of infection. Flow cytometric analysis of GFP routinely revealed that greater than 70% of cells were virally infected.
Confocal microscopic analysis
Cells were fixed with 100% cold methanol for 10 minutes. Nonspecific protein binding was prevented by blocking the cells with 5% fetal calf serum in PBS for 30 minutes. Cells were stained with the appropriate primary antibodies (1:200 dilution) for 2 hours at room temperature or overnight at 4°C. After 3 washes with PBS, cells were stained with the appropriate anti–donkey secondary antibodies (labeled with fluorescein isothiocyanate or Texas Red 1:200 dilution) for 45 minutes and washed with PBS. Coverslips were applied with Slow Fade reagent (Molecular Probes). The cells were visualized by a FluoView 500 (FV500) laser scanning confocal microscope (Olympus America). Images were captured with a PlanApo 60×/1.4 oil objective using the appropriate filter sets. Digital images were obtained using the manufacturer's FluoView v.5.0 software. Image resolutions were adjusted using Adobe Photoshop CS3.
Results
Model system for studying the functional role of NFATc1 in DLBCL
A recent study showed that NFATc1 regulates c-myc transcription in pancreatic cancers.14 To examine the possible correlation of NFATc1 and c-myc protein expression in aggressive NHL-B, 8 cell lines (a well-known Burkitt lymphoma cell line Ramos and 7 of our DLBCL cell lines) were examined for expression of NFATc1 and c-myc proteins by Western blot analysis. As shown in Figure 1A, expression of the c-myc protein correlated with nuclear NFATc1 protein expression in all cell lines examined. One DLBCL cell line (DS) was negative for both NFATc1 and c-myc protein expression. The mRNA level of c-myc was shown to correlate with the DNA binding activity of NFATc1 in representative DLBCL cell lines (P = .166; Figure 1B). Similar results were obtained from an NFAT and c-myc TMA in 100 representative DLBCL cases. NFATc1 and c-myc TMA slides containing 100 DLBCL cases, as well as examples for positive and negative NFATc1 and c-myc immnostaining are shown in Figure 1C. Seventy-two percent of DLBCL cases showed positive staining (> 30% positive cells) for both NFATc1 and c-myc expression (Figure 1D). Seven cases showed negative staining for c-myc that also showed negative for NFATc1 staining. Of the 72 cases that were positive staining for NFATc1 staining, 59 (82%) had nuclear NFATc1 staining as well as c-myc nuclear staining. NFATc1 is also highly expressed in reactive lymph node tissue, but mostly in the cytoplasmic compartment with very little nuclear staining (Figure 1E). These findings suggest that c-myc is a likely NFATc1 target gene in aggressive NHL-B cells, and that nuclear NFATc1 could play an important role in the biology of NHL-B.
Correlation of NFATc1 and c-myc protein expression in B-cell lymphomas. (A) Nuclear extracts (25 μg) were purified from a Burkitt lymphoma cell lines (Ramos) and 7 DLBCL cell lines (BJAB,LR, McA, DS, MS, LP, and EJ) and analyzed for expression of NFATc1, c-myc, and Oct-1 (protein loading control) proteins by Western blotting. *, **, and *** denote hyperphosphorylated, phosphorylated, and dephosporylated NFATc1 protein bands, respectively. (B) Purified RNA and nuclear extracts from representative DLBCL cell lines were analyzed for c-myc mRNA expression and for NFATc1 DNA binding, respectively. DS, negative for both c-myc and NFATc1, was used as a baseline negative control. Values indicate fold-induction over DS from triplicate samples of 2 independent experiments. SU4, SUDHL-4; LY10, OCI-LY10. P for NFATc1 DNA binding versus c-myc mRNA for all cell lines was determined by the Student t test (P = .166; R2 = 0.07246). (C) TMA of DLBCL (100 cases) for NFATc1 and c-myc protein expressions by immunohistochemistry. Representative high-magnification (×200) TMA sections from DLBCL biopsy cores that are negative (−, black box), moderate (+, green box) or high (++, red box) immunostaining for NFATc1 and c-myc (side panels). (D) Analysis for NFATc1 and c-myc protein expression in DLBCL TMA (100 cases), using 30% cutoff. (E) Immunostaining for NFATc1 in reactive lymph node section. GC, germinal center (×100; ×400).
Correlation of NFATc1 and c-myc protein expression in B-cell lymphomas. (A) Nuclear extracts (25 μg) were purified from a Burkitt lymphoma cell lines (Ramos) and 7 DLBCL cell lines (BJAB,LR, McA, DS, MS, LP, and EJ) and analyzed for expression of NFATc1, c-myc, and Oct-1 (protein loading control) proteins by Western blotting. *, **, and *** denote hyperphosphorylated, phosphorylated, and dephosporylated NFATc1 protein bands, respectively. (B) Purified RNA and nuclear extracts from representative DLBCL cell lines were analyzed for c-myc mRNA expression and for NFATc1 DNA binding, respectively. DS, negative for both c-myc and NFATc1, was used as a baseline negative control. Values indicate fold-induction over DS from triplicate samples of 2 independent experiments. SU4, SUDHL-4; LY10, OCI-LY10. P for NFATc1 DNA binding versus c-myc mRNA for all cell lines was determined by the Student t test (P = .166; R2 = 0.07246). (C) TMA of DLBCL (100 cases) for NFATc1 and c-myc protein expressions by immunohistochemistry. Representative high-magnification (×200) TMA sections from DLBCL biopsy cores that are negative (−, black box), moderate (+, green box) or high (++, red box) immunostaining for NFATc1 and c-myc (side panels). (D) Analysis for NFATc1 and c-myc protein expression in DLBCL TMA (100 cases), using 30% cutoff. (E) Immunostaining for NFATc1 in reactive lymph node section. GC, germinal center (×100; ×400).
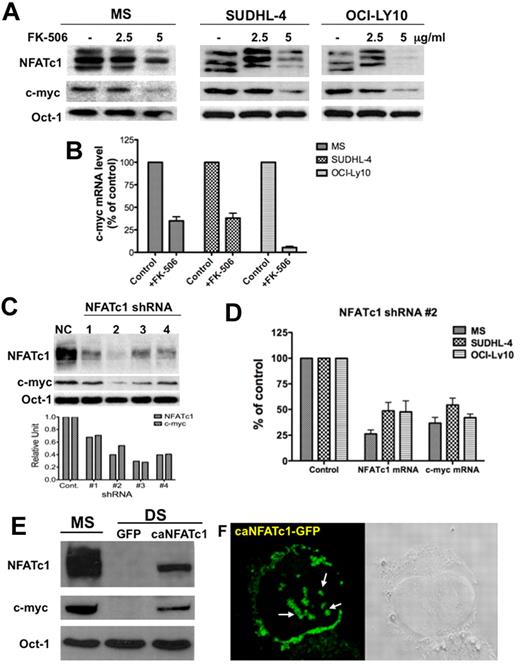
Next, we suppressed NFAT activation in representative DLBCL cell lines (which express NFATc1 at high levels) with an NFAT inhibitor, FK-506, and then examined the levels of c-myc protein and mRNA. Treatment with FK-506 down-regulated both dephosphorylated NFATc1 (active form) and c-myc protein (Figure 2A) as well as c-myc mRNA (Figure 2B) expression in these cells. Moreover, specific down-regulation of NFATc1 by small hairpin RNA (shRNA) in MS cells also inhibited c-myc protein expression (Figure 2C). Knockdown of NFATc1 by shRNA also down-regulated c-myc mRNA expression in representative DLBCL cell lines (Figure 2D). Conversely, transduction of DLBCL DS cells (negative for both NFATc1 and c-myc) with a retroviral vector containing a constitutively active mutant of NFATc1 induced c-myc protein expression (Figure 2C). Constitutively active NFATc1 in DS cells appears as punctated dots in both the cytoplasm as well as in the nuclear compartment (Figure 2D). These results suggest that c-myc is a potential target gene for NFATc1 transcription in aggressive B-cell lymphomas.
NFATc1 regulates c-myc protein expression in DLBCL. (A) DLBCL MS, SUDHL4 and OCI-LY10 cells were treated with FK-506 (2.5 and 5 μg/mL) for 24 hours. Nuclear extracts (25 μg) were subjected to Western blotting for NFATc1, c-myc, and Oct-1 (loading control). (B) Purified RNA from control and FK-506 treated cells from part A was analyzed for c-myc mRNA expression. (C) DLBCL MS cells were transfected with a control shRNA (NC) or NFATc1 shRNA no. 1-4. Forty-eight hours after transfection, cells were harvested and nuclear extracts were purified. Nuclear extracts (25 μg) were subjected to Western blotting for NFATc1, c-myc, and Oct-1 (loading control). Densitometry analysis was performed for NFATc1 and c-myc protein bands (bottom graph). (D) DLBCL MS, SUDHL4, and OCI-LY10 cells were transfected with a control shRNA or an NFATc1 no. 2 shRNA. Forty-eight hours after transfection, purified RNA was analyzed for NFATc1 and c-myc mRNA expressions. (E) Exogenous expression of NFATc1 induces c-myc protein expression. DLBCL DS cells were infected with a retroviral vector containing a mutant NFATc1 (caNFATc1-GFP). After 48 hours of incubation, nuclear extracts (25 μg) were purified and analyzed for expression of NFATc1, c-myc, and Oct-1 proteins by Western blotting. (F) NFATc1-GFP fusion protein expression in infected DS cells was analyzed by confocal microscopic analysis, which indicates punctate nuclear and cytoplasmic expression of dephosphorylated NFATc1.
NFATc1 regulates c-myc protein expression in DLBCL. (A) DLBCL MS, SUDHL4 and OCI-LY10 cells were treated with FK-506 (2.5 and 5 μg/mL) for 24 hours. Nuclear extracts (25 μg) were subjected to Western blotting for NFATc1, c-myc, and Oct-1 (loading control). (B) Purified RNA from control and FK-506 treated cells from part A was analyzed for c-myc mRNA expression. (C) DLBCL MS cells were transfected with a control shRNA (NC) or NFATc1 shRNA no. 1-4. Forty-eight hours after transfection, cells were harvested and nuclear extracts were purified. Nuclear extracts (25 μg) were subjected to Western blotting for NFATc1, c-myc, and Oct-1 (loading control). Densitometry analysis was performed for NFATc1 and c-myc protein bands (bottom graph). (D) DLBCL MS, SUDHL4, and OCI-LY10 cells were transfected with a control shRNA or an NFATc1 no. 2 shRNA. Forty-eight hours after transfection, purified RNA was analyzed for NFATc1 and c-myc mRNA expressions. (E) Exogenous expression of NFATc1 induces c-myc protein expression. DLBCL DS cells were infected with a retroviral vector containing a mutant NFATc1 (caNFATc1-GFP). After 48 hours of incubation, nuclear extracts (25 μg) were purified and analyzed for expression of NFATc1, c-myc, and Oct-1 proteins by Western blotting. (F) NFATc1-GFP fusion protein expression in infected DS cells was analyzed by confocal microscopic analysis, which indicates punctate nuclear and cytoplasmic expression of dephosphorylated NFATc1.
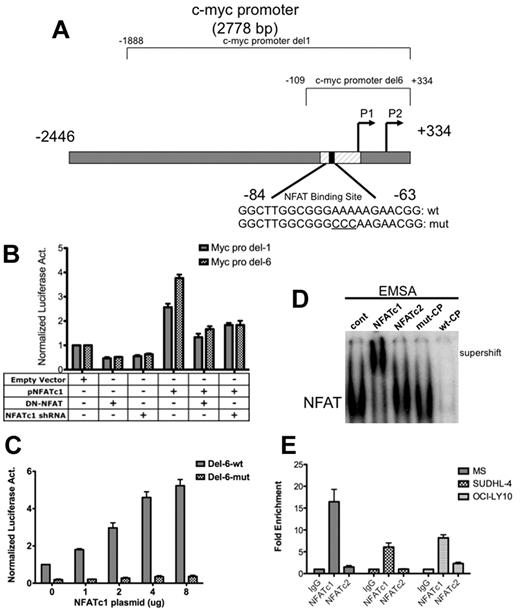
To test whether NFATc1 does in fact, act as a transcriptional regulator of c-myc, DLBCL MS cells were cotransfected with a c-myc luciferase reporter plasmid containing either a full-length c-myc promoter comprising positions −2446 to +334 (Del-1) or a c-myc promoter fragment containing positions −109 to +334 (Del-6) relative to the P2 transcription start site, both of which contain an NFAT site (Figure 3A), along with an NFATc1 expression plasmid in combination with an NFATc1 shRNA expression plasmid or a dominant-negative NFAT expression construct. The results of these experiments showed that NFATc1 enhanced c-myc promoter activity in DLBCL cells and that this promoter activity can be repressed by cotransfecting the cells with a dominant-negative NFAT plasmid or reduced by cotransfection with NFATc1 shRNA (Figure 3B). The c-myc promoter fragment Del-6 had luciferase activity similar to that of the full-length promoter (Figure 3B), and NFATc1 enhanced Del-6 promoter activity in a concentration-dependent manner (Figure 3C), suggesting that NFATc1 plays a prominent role in regulation of the proximal c-myc promoter in DLBCL. The proximal c-myc promoter (Del-6) contains a motif that binds to NFATc1 in pancreatic cells.14 Mutating this NFAT site diminished the NFATc1-induced c-myc promoter activity (Figure 3C). To determine whether NFATc1 binds to this binding site in lymphoma B cells, nuclear extracts were purified from MS cells and analyzed for DNA binding by gel-shift assays. As shown in Figure 3D, supershift electromobility gel-shift assay shows that the predominant DNA binding activity was provided by NFATc1, but negative binding was observed with the other NFAT family member, NFATc2. ChIP assays followed by Q-PCR further confirmed binding of NFATc1 to the c-myc promoter in representative DLBCL cell lines (Figure 3E). These results suggest that NFATc1 binds directly to the proximal end of the c-myc promoter and up-regulates c-myc gene transcription in lymphoma B cells. Our previous studies had indicated that, in addition to c-myc, NFATc1 also regulates 2 key growth and survival genes, CD40L and BLyS, in DLBCL. These studies indicate that these DLBCL cell lines (MS and DS) are useful models for studying transcriptional regulation of c-myc in defining the functional role of NFATc1 in transcriptional regulation of the important growth and survival genes in B-cell lymphomas.
NFATc1 binds to and regulates c-myc promoter in DLBCL. (A) Schematic diagram of the c-myc promoter. c-myc Del-1 and Del-6 are luciferase reporter constructs obtained from Addgene. (B) DLBCL MS cells were cotransfected with a c-myc Del-1 or Del-6 construct (2 μg) and an empty vector (5 μg) or a plasmid containing NFATc1 (5 μg, alone or combined with dominant-negative NFAT or NFATc1 shRNA). The NFATc1 shRNA is retained in the NFATc1 expression construct. Luciferase activities were analyzed 24 hours after transfection. Data indicate fold-induction compared with empty vector alone. Luciferase activities were normalized with β-gal activity. Data represent 3 independent experiments. (C) DLBCL MS cells were cotransfected with c-myc promoter Del-6 (2 μg) or c-myc promoter Del-6 with a mutated NFAT binding site (Del-6 mut; 2 μg) with increasing NFATc1 plasmid, as indicated. Luciferase activities were measured 24 hours after transfection. Luciferase activities were normalized with β-gal activity. Data represent 3 independent experiments. (D) DLBCL MS nuclear extracts were subjected to gel-shift assays using the 32P-labeled NFAT-binding site within the proximal end of the c-myc promoter. NFATc1 and NFATc2 antibodies were used for supershift. wt-CP, wild-type cold probe; mut-CP, mutant cold probe. (E) ChIP analysis in MS, SUDHL-4, and OCI-LY10 cells using antibodies to NFATc1, NFATc2, and IgG (negative control), followed by Q-PCR of the c-myc promoter (NFAT binding site). Input (10% of total DNA). Values indicate fold-enrichment over IgG control from 2 independent experiments with triplicate samples.
NFATc1 binds to and regulates c-myc promoter in DLBCL. (A) Schematic diagram of the c-myc promoter. c-myc Del-1 and Del-6 are luciferase reporter constructs obtained from Addgene. (B) DLBCL MS cells were cotransfected with a c-myc Del-1 or Del-6 construct (2 μg) and an empty vector (5 μg) or a plasmid containing NFATc1 (5 μg, alone or combined with dominant-negative NFAT or NFATc1 shRNA). The NFATc1 shRNA is retained in the NFATc1 expression construct. Luciferase activities were analyzed 24 hours after transfection. Data indicate fold-induction compared with empty vector alone. Luciferase activities were normalized with β-gal activity. Data represent 3 independent experiments. (C) DLBCL MS cells were cotransfected with c-myc promoter Del-6 (2 μg) or c-myc promoter Del-6 with a mutated NFAT binding site (Del-6 mut; 2 μg) with increasing NFATc1 plasmid, as indicated. Luciferase activities were measured 24 hours after transfection. Luciferase activities were normalized with β-gal activity. Data represent 3 independent experiments. (D) DLBCL MS nuclear extracts were subjected to gel-shift assays using the 32P-labeled NFAT-binding site within the proximal end of the c-myc promoter. NFATc1 and NFATc2 antibodies were used for supershift. wt-CP, wild-type cold probe; mut-CP, mutant cold probe. (E) ChIP analysis in MS, SUDHL-4, and OCI-LY10 cells using antibodies to NFATc1, NFATc2, and IgG (negative control), followed by Q-PCR of the c-myc promoter (NFAT binding site). Input (10% of total DNA). Values indicate fold-enrichment over IgG control from 2 independent experiments with triplicate samples.
Involvement of the chromatin-remodeling complex protein Brg-1 with NFATc1 within the c-myc promoter
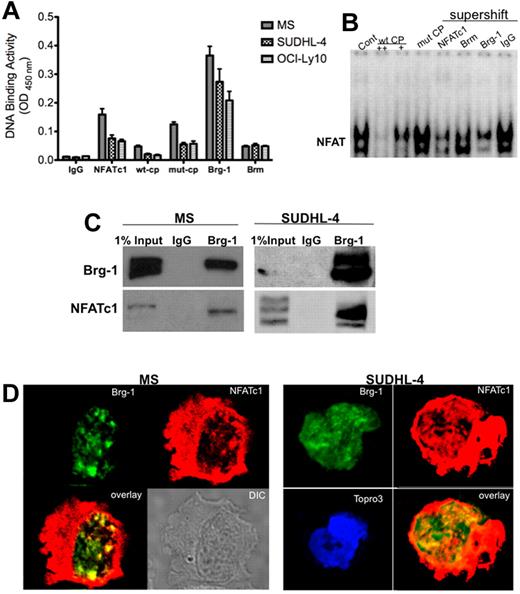
The transcription factor NFATc1 is known for its active role in forming enhanceosomes and was recently recognized as a nucleosome reorganization factor involved with the chromatin remodeling complex proteins.28 To examine whether NFATc1 is involved in the chromatin remodeling mechanism in lymphoma B cells, we tested the direct association of NFATc1 protein and the chromatin remodeling complex ATP enzymes Brm and Brg-1 using DNA-binding ELISAs. In assays using nuclear extracts purified from representative DLBCL cell lines, both Brm and Brg-1 proteins bound to the consensus NFAT-binding sequence (Figure 4A). Because Brg-1 bound to the NFAT-binding site with higher intensities than Brm, our remaining studies focused primarily on Brg-1 and its activity in mediating NFATc1 function. Next, we used gel-shift assays to test whether Brg-1 bound to the NFAT-binding site within the c-myc promoter. Figure 4B shows that antibodies against Brg-1 competed out the NFAT-binding complex, suggesting that Brg-1 proteins are part of the NFATc1 complex in DLBCL cells. Further analysis by immunoprecipitation assays showed that Brg-1 interacts directly with NFATc1 (Figure 4C), and confocal microscopic analysis confirmed that Brg-1 and NFATc1 colocalized in the nuclear compartment in punctate nuclear complexes (Figure 4D). These results indicate a direct link between the transcription factor NFATc1 and the chromatin remodeling complex protein Brg-1 in DLBCL and suggest that NFATc1 can function actively in chromatin remodeling mechanism.
NFATc1 involved in chromatin remodeling mechanism. Chromatin remodeling complex proteins Brg-1 and Brm bind to the NFAT site in the c-myc promoter. (A) Nuclear extracts purified from MS, SUDHL-4, and OCI-LY10 DLBCL cells were analyzed for NFATc1, Brg-1, and Brm DNA binding to the NFAT consensus binding site by DNA-binding ELISA. IgG was used as a nonspecific control antibody. wt-CP, wild-type cold probe; mut-CP, mutant cold probe. (B) DLBCL MS nuclear extracts were subjected to gel-shift assay using the 32P-labeled c-myc promoter NFAT DNA-binding site and antibodies to NFATc1, NFATc2, Brg-1, Brm, p65, and c-rel. wt-CP, wild-type cold probe; mut-CP, mutant cold probe. (C) Brg-1 interacts directly with NFATc1. DLBCL MS nuclear extracts (500 μg) or SUDHL-4 nuclear extracts (1 mg) were purified and subjected to coimmunoprecipitation analysis with IgG control or Brg-1 antibody. Eluted immunoprecipitated protein complexes were subjected to Western blotting for Brg-1 and NFATc1. (D) Colocalization of Brg-1 (green) and NFATc1 (red) in DLBCL MS and SUDHL-4 cells by confocal microscopy analysis. Colocalized areas appear yellow. Topro3, nuclear marker.
NFATc1 involved in chromatin remodeling mechanism. Chromatin remodeling complex proteins Brg-1 and Brm bind to the NFAT site in the c-myc promoter. (A) Nuclear extracts purified from MS, SUDHL-4, and OCI-LY10 DLBCL cells were analyzed for NFATc1, Brg-1, and Brm DNA binding to the NFAT consensus binding site by DNA-binding ELISA. IgG was used as a nonspecific control antibody. wt-CP, wild-type cold probe; mut-CP, mutant cold probe. (B) DLBCL MS nuclear extracts were subjected to gel-shift assay using the 32P-labeled c-myc promoter NFAT DNA-binding site and antibodies to NFATc1, NFATc2, Brg-1, Brm, p65, and c-rel. wt-CP, wild-type cold probe; mut-CP, mutant cold probe. (C) Brg-1 interacts directly with NFATc1. DLBCL MS nuclear extracts (500 μg) or SUDHL-4 nuclear extracts (1 mg) were purified and subjected to coimmunoprecipitation analysis with IgG control or Brg-1 antibody. Eluted immunoprecipitated protein complexes were subjected to Western blotting for Brg-1 and NFATc1. (D) Colocalization of Brg-1 (green) and NFATc1 (red) in DLBCL MS and SUDHL-4 cells by confocal microscopy analysis. Colocalized areas appear yellow. Topro3, nuclear marker.
NFATc1 recruits Brg-1 and other transcription factors to the c-myc promoter
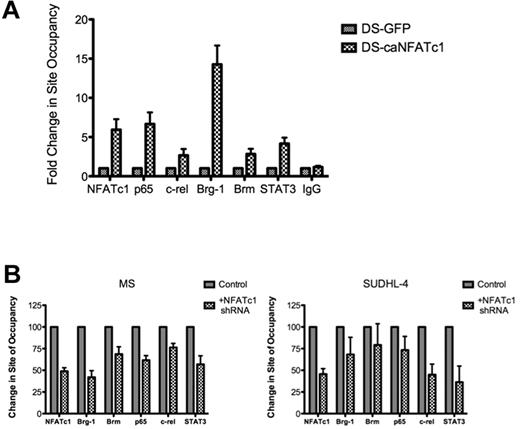
We then examined whether NFATc1 was involved in recruiting Brg-1 and other transcription factors to the NFATc1-targeted gene promoters. Ectopic expression of a constitutively active mutant of NFATc1 in DLBCL DS cells (negative for NFATc1) was sufficient to recruit Brg-1 as well as other transcription factors, such as NF-κB-p65, c-rel, and STAT3, to the NFAT DNA-binding site in the c-myc promoter (Figure 5A). Conversely, inhibiting NFATc1 in DLBCL MS and SUDHL-4 cells with shRNA diminished Brg-1 and other transcription factors binding to the c-myc gene promoters (Figure 5B).
Recruitment of Brg-1 and other factors to the c-myc promoter by NFATc1. (A) DLBCL DS cells (NFATc1−) were infected with a retroviral vector containing a mutant NFATc1 (caNFATc1-GFP) or vector containing only GFP (negative control). After 48 hours of incubation, cells were fixed and analyzed for protein binding to the NFAT DNA-binding site in the c-myc promoter by ChIP–Q-PCR with antibodies to NFATc1, p65, c-rel, Brg-1, Brm, STAT3, and IgG (negative control). The data were analyzed by the ChIP–Q-PCR Data Analysis Template. Data represent 3 independent experiments. (B) DLBCL MS and SUDHL-4 cells (NFATc1+) were transfected with the NFATc1 shRNA plasmid or control vector. Forty-eight hours after transfection, cells were fixed and analyzed for protein binding to the NFAT DNA-binding site in the c-myc promoter by ChIP–Q-PCR with antibodies to NFATc1, p65, c-rel, Brg-1, Brm, STAT3, and IgG (negative control). The data were analyzed by the SuperArray ChIP–Q-PCR Data Analysis Template. Data represent 3 independent experiments.
Recruitment of Brg-1 and other factors to the c-myc promoter by NFATc1. (A) DLBCL DS cells (NFATc1−) were infected with a retroviral vector containing a mutant NFATc1 (caNFATc1-GFP) or vector containing only GFP (negative control). After 48 hours of incubation, cells were fixed and analyzed for protein binding to the NFAT DNA-binding site in the c-myc promoter by ChIP–Q-PCR with antibodies to NFATc1, p65, c-rel, Brg-1, Brm, STAT3, and IgG (negative control). The data were analyzed by the ChIP–Q-PCR Data Analysis Template. Data represent 3 independent experiments. (B) DLBCL MS and SUDHL-4 cells (NFATc1+) were transfected with the NFATc1 shRNA plasmid or control vector. Forty-eight hours after transfection, cells were fixed and analyzed for protein binding to the NFAT DNA-binding site in the c-myc promoter by ChIP–Q-PCR with antibodies to NFATc1, p65, c-rel, Brg-1, Brm, STAT3, and IgG (negative control). The data were analyzed by the SuperArray ChIP–Q-PCR Data Analysis Template. Data represent 3 independent experiments.
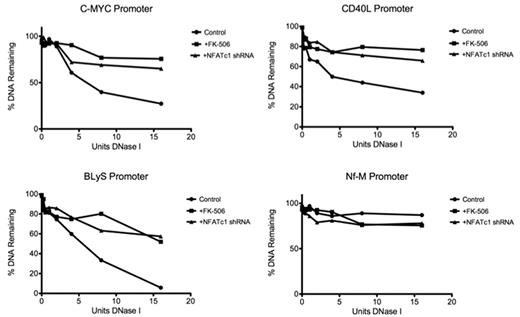
NFATc1 induces DNase I hypersensitive sites in NFAT-targeted gene promoters
Because NFATc1 is able to recruit chromatin remodeling complex protein Brg-1 and other transcription factors to chromatin, we hypothesized that an NFATc1/Brg-1 complex could play a prominent role in modulating NFATc1 target genes by inducing DHS, which leads to an open chromatin structure accessible to transcription factors. The c-myc promoter has been well mapped for DHS and has a prominent DHS within the NFAT-binding site.29 To determine whether NFATc1 is in fact involved in forming DHS in DLBCL, we quantitated DNase I hypersensitivity in promoters of NFATc1-targeted genes by treating with DNase I, followed by Q-PCR. The results show that the DNA regions containing the NFAT-binding site within the c-myc promoter, as well as in other NFATc1-targeted gene promoters such as CD40L and BLyS, are hypersensitive to DNase I treatment (Figure 6). However, the same DNA regions were insensitive to DNase I treatment when NFATc1 was down-regulated, either with FK-506 treatment or NFATc1 shRNA transfection (Figure 6), suggesting that NFATc1 plays a prominent role in DHS formation in DLBCL.
NFATc1 induces DNase I in target gene promoters in DLBCL. Plots of the DNase I digestion of control and FK-506-treated or NFATc1 shRNA-transfected DLBCL MS cells for promoters of c-myc, CD40L, BLyS, and Nf-M (a known DNase I–resistant locus). Standard curves were used to calculate the percentage of copies of the promoter's amplicon remaining in 50 ng of DNase I–treated genomic DNA.
NFATc1 induces DNase I in target gene promoters in DLBCL. Plots of the DNase I digestion of control and FK-506-treated or NFATc1 shRNA-transfected DLBCL MS cells for promoters of c-myc, CD40L, BLyS, and Nf-M (a known DNase I–resistant locus). Standard curves were used to calculate the percentage of copies of the promoter's amplicon remaining in 50 ng of DNase I–treated genomic DNA.
Discussion
NFAT family proteins are multifunctional activators, and also important calcium-inducible transcription factors that activate expression of a wide range of immune response genes, especially in activated T lymphoid cells,30 but our previous studies have indicated that NFAT proteins are also important in B lymphocytes, and particularly in aggressive human NHL-B.5,19 Recent experimental evidence has revealed a key role for NFAT transcription factors in human tumor progression, particularly in hematologic malignancies. In this study, we report that NFATc1 regulates c-myc as well as other growth and survival genes in DLBCL through a chromatin remodeling mechanism involving the recruitment of the chromatin remodeling ATPase enzyme Brg-1. These findings suggest that nuclear NFATc1 is intrinsically involved in the pathophysiology of DLBCL and pharmacologic interdiction of this pathway could provide new therapeutic avenues for clinical intervention.
NFAT-dependent promoters and enhancers rapidly undergo extensive inducible chromatin remodeling to form DHS.31,32 NFAT is likely to be a driving force behind this chromatin remodeling, which has recently been proposed as a major NFAT function,28 because it has been shown that NFAT sites alone are sufficient to activate DHS in a chromatin context. Chromatin remodeling may well be a primary function of NFAT elements, because even high-affinity NFAT sites are relatively weak transcriptional activators in the absence of the collaborating transcription factors with which they normally associate.17 Transcription factor–activating protein 1 (AP-1) is the most common partner directly recruited by NFAT, at the level of DHS, and composite NFAT–AP-1 DNA response elements are very efficient in evicting nucleosomes.32 NFAT–AP-1 complexes recruit both histone acetyltransferases (HATs) and the ATP-dependent SWI-SNF family of chromatin remodelers,33,34 that together provide the functional proteins needed to modify and rearrange nucleosomes.
Recent studies have shown that NFAT may also help to organize chromatin domains and enable enhancer-promoter communication.35,36 In activated T cells, inducible intrachromosomal looping occurs between the tumor necrosis factor (TNF) gene promoter and 2 NFAT-dependent enhancers located at −9 kb and +3 kb relative to the start site of transcription.18 This topology places the TNF gene and the adjacent lymphotoxin genes in separate loops, thereby allowing independent regulation of the TNF gene within a multigene locus. These new data support earlier studies indicating that NFAT functions through the disruption of nucleosomes within specific gene enhancers, mobilizing nucleosomes across extensive chromatin domains linking enhancers and promoters. Our studies identify NFAT as a factor in DLBCL cells, that creates a chromatin environment that is permissive for both recruitment and aggregation of factors that control transcriptional processes within important targeted promoters and enhancers.37 The specific role that NFAT plays in the complex process of gene locus activation is still unclear, but it is clearly an effective facilitator for initiating the first essential step of creating a critical accessible “open” chromatin environment,28 that we have shown to function in NFAT-targeted G/S survival genes in DLBCL.
The consequence of an active NFAT transcription factor playing a role in chromatin remodeling in regulating important growth and survival genes, such as c-myc, could be beneficial to many cancer cells, including B-cell lymphomas. Previous studies have shown that deregulated c-myc expression due to alternative myc translocation, amplification, or mutation is a negative prognostic indicator in DLBCL.38 Constitutive NFAT activation in DLBCL, on the other hand, could be one plausible molecular mechanism for maintaining continuous c-myc expression. In fact, c-myc can control the NFAT pathway in B lymphocytes by directly amplifying the calcium signal that leads to sustained intracellular calcium and maintaining continual NFAT nuclear translocation, thereby enabling concurrent expression of Myc- and calcium-regulated target genes.39 In this sense, deregulated myc or NFATc1 in DLBCL could provide a positive regulatory feedback loop in which NFATc1 and c-myc mutually reinforce each other's expression, subsequently maintaining neoplastic cell growth and survival. Recent studies have already established that NFATc1 is a key transcription factor involved in cell growth and survival as well as cellular transformation in vitro in various cell types.5,11,15,16,19 However, the consequence of an active NFATc1 in an in vivo genetic-engineered mouse model setting has not been demonstrated and warrants further investigation.
We discovered that the chromatin remodeling ATPase dependent enzyme Brg-1 binding to growth and survival gene promoters is dependent on the direct interaction with NFATc1 in DLBCL. Brg-1 has been shown to unfold or displace nucleosomes to create “open” chromatin structures.40 Therefore, we hypothesized that once Brg-1 is recruited and interacts with NFATc1 at the chromatin loci, these factors are able to unfold these chromatin loci to create DNase I hypersensitive sites, resulting in transcriptionally active and accessible chromatin, allowing other transcription factors to bind to these sites. Although chromatin remodeling proteins have been implicated in the oncogenic transformation,41 the underlying mechanisms are still poorly understood. SNF5, a core subunit of the SWI/SNF complex, is a potent tumor suppressor that is specifically inactivated in several types of human cancer.42,43 A recent study showed that lymphomagenesis in the absence of SNF5 does not result from SWI/SNF inactivation but rather that oncogenesis is dependent on continued presence of Brg-1.44 These findings along with our current findings suggest that Brg-1 plays an important role in the biology of B-cell lymphomas and that targeted inhibition of Brg-1 ATPase activity could provide a novel and effective therapeutic approach for aggressive B-cell lymphomas.
In summary, this study has shown a key function for the transcription factor NFATc1 in regulating important growth and survival genes (ie, c-myc, CD40L, BlyS) in DLBCL through an epigenetic chromatin remodeling mechanism that requires recruitment of the ATPase enzyme Brg-1. Our finding that NFATc1 is a component of a chromatin remodeling complex not only extends these findings by implicating chromatin remodeling complexes in the pathogenesis of DLBCL, but also provides a mechanistic insight into NFATc1 function and the biochemical pathways regulated by NFATc1. These results also suggest that targeting the NFAT/Brg-1 pathway in DLBCL could have therapeutic potential, particularly for patients with relapsed or refractory DLBCL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Odyssey Program and the Kimberly-Clark Foundation Award for Scientific Achievement at The University of Texas M. D. Anderson Cancer Center (to L.V.P.). This work was also supported by National Cancer Institute grants CA-RO1-100836 (to R.J.F.) and CA-16672-26 (Cancer Center Support Grant), and a grant from the Leukemia & Lymphoma Society (to R.J.F.).
National Institutes of Health
Authorship
Contribution: L.V.P. and R.J.F. contributed to all scientific aspects of the manuscript (designed and performed research and analyzed data) and wrote the paper; A.T.T. and C.L. contributed by performing experiments and analyzing data; and C.B.-R. contributed the immunohistochemical analysis.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
Correspondence: Lan V. Pham, Department of Hematopathology, Box 54, The University of Texas of M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: lvpham@mdanderson.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal