Abstract
Approximately 3% of all human T-lymphotropic virus type 1 (HTLV-1)–infected persons will develop a disabling inflammatory disease of the central nervous system known as HTLV-1–associated myelopathy/tropical spastic paraparesis, against which there is currently no efficient treatment. As correlation exists between the proviral load (PVL) and the clinical status of the carrier, it is thought that diminishing the PVL could prevent later occurrence of the disease. We have conducted a study combining valproate, an inhibitor of histone deacetylases, and azidothymidine, an inhibitor of reverse transcriptase, in a series of baboons naturally infected with simian T-lymphotropic virus type 1 (STLV-1), whose PVL was equivalent to that of HTLV-1 asymptomatic carriers. We show that the combination of drugs caused a strong decrease in the PVL and prevented the transient rise in PVL that is seen after treatment with histone deacetylases alone. We then demonstrate that the PVL decline was associated with an increase in the STLV-1–specific cytotoxic T-cell population. We conclude that combined treatment with valproate to induce viral expression and azidothymidine to prevent viral propagation is a safe and effective means to decrease PVL in vivo. Such treatments may be useful to reduce the risk of HAM/TSP in asymptomatic carriers with a high PVL.
Introduction
Ten million to 20 million people are infected with human T-lymphotropic virus type 1 (HTLV-1) worldwide. This retrovirus is the etiologic agent of a malignant lymphoproliferative disease called adult T-cell leukemia/lymphoma (ATLL),1,2 and of several inflammatory diseases, particularly of HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP).3-5 Despite numerous clinical trials, no efficient treatment has yet been described for HAM/TSP patients. HAM/TSP is a neurodegenerative disease with major irreversible demyelination and loss of motor neurons from the pyramidal tract,6-11 and the current treatments can only stabilize the clinical status of the patient. It is therefore necessary to develop therapies that can prevent the occurrence of this virus-associated neurodegenerative disease or at least block the evolution of the disease in the early stages of the pathogenesis.
HTLV-1 infects a wide range of cells in vitro. However, in vivo, it is principally detected in CD4+ T lymphocytes and, to a lesser extent, CD8+ cells.12-16 Importantly, there is an association between the HTLV-1 proviral load (PVL) and the clinical status of the persons. The PVL measured in the peripheral blood mononuclear cells (PBMCs) isolated from HAM/TSP patients is 5- to 20-fold higher than that of asymptomatic carriers (ACs).17-21 Similarly, human leukocyte antigen genotypes associated with a low PVL are associated with a reduced risk of HAM/TSP.22,23 It is therefore probable that the PVL in an AC will predict the risk of developing an HTLV-1-related disease. In ACs with a high PVL, diminishing the PVL may also prevent later occurrence of the disease.
Most cells infected with HTLV-1 appear transcriptionally silent at a given time, which therefore makes viral expression difficult to detect in vivo in ACs, even when using highly sensitive techniques, such as reverse-transcriptase polymerase chain reaction (PCR).24,25 It is known that the virus replicates mostly during the chronic phase of infection via clonal expansion of the infected cells.26 This pseudo-latency renders HTLV-1 resistant to antiretroviral drug treatments in vivo, such as azidothymidine (AZT), and probably plays an important role in viral escape from the host immune response.24,27 As with cellular genes, the expression of the HTLV-1 provirus is epigenetically regulated, and the low level of viral expression is associated with proviral chromatin deacetylation and condensation.28,29 A transient transcriptional activation of the viral reservoir should therefore allow a reduction of the PVL in vivo, as long as the host maintains an efficient antiviral immune response.
A recent in vitro study demonstrated that treating HTLV-1–infected lymphocytes with valproate (VPA), an inhibitor of histone deacetylases (HDACi), induces viral expression and cell death.30,31 A clinical trial with VPA was then initiated in a series of 16 HAM/TSP patients at a late development stage of the disease.9 This led to a decrease of the PVL after 45 to 60 days of treatment.9,32 Unfortunately, no significant improvement was observed in the clinical status of the patients, perhaps because the HTLV-1–associated neurologic damage had already taken place or because a longer follow-up period was necessary. Moreover, a transient PVL rise was consistently observed in the first weeks of the treatment, probably because of the induction of HTLV-1 proviral expression by VPA and subsequent infectious propagation. Because PVL is linked to disease progression, this observation represents a major concern that currently prevents further use of VPA-based therapy.33 In this study, the PVL rise was associated with an increase in the 8-m walking time. It was also pointed out that, in theory, the transient PVL peak might favor central nervous system invasion (and thus worsen inflammation), as it potentially increases the probability that an HTLV-1–infected lymphocyte will cross the blood-brain barrier.32 Therefore, preventing the PVL increase at the initiation of treatment should be beneficial in the long-term. This trial suggested that VPA might constitute an effective means to prevent or treat HAM/TSP if one could prevent the initial viral replication.
We therefore conducted a study combining VPA and AZT, an inhibitor of the reverse transcriptase, in a series of baboons (Papio papio) naturally infected with simian T-lymphotropic virus type 1 (STLV-1). STLV-1 is the simian counterpart of HTLV-1. The 2 viruses are almost identical at the nucleotide sequence level. Baboons constitute an interesting, but little-used, model of asymptomatic HTLV-1 infection34 : their immune system is very similar to the human, the animals are naturally infected with STLV-1, and some develop STLV-1-associated diseases, such as ATLL.35 We report here that the VPA/AZT combined treatment induced a strong decrease in the PVL in treated animals and prevented the transient rise in PVL observed after treatment with HDACi alone. The PVL decline was correlated with an increase in the STLV-1-specific cytotoxic T-cell population.
Methods
Animal treatment
Fourteen baboons (Papio papio), naturally infected with STLV-1, were included in this study. Animals were housed at the primate center of the Centre National de la Recherche Scientifique in Rousset-sur-Arc and cared for in compliance with French regulations. The Regional Committee on Animal Experimentation approved this study. STLV-1 infection was initially determined by serologic methods and confirmed by PCR (data not shown). Three animals were left untreated, 4 animals received VPA alone (Depakine chrono, Sanofi Aventis; 500 mg/day), 2 animals received AZT alone (Retrovir, GlaxoSmithKline; 300 mg/day), and 5 received a combination of both drugs (Table 1). The drugs were given orally (embedded in food such as bananas) to each baboon on a daily basis. In all cases, veterinarians ascertained that the drug had been eaten. This was also confirmed by measuring the VPA serum levels for each blood sample, using a routine diagnostic method (data not shown). Treatment toxicity was monitored by behavioral observations, regular weight measurement, and transaminase blood level assessment (aspartate aminotransferase and alanine aminotransferase; data not shown). The treatment lasted 2 months. Peripheral venous blood samples from 5 noninfected baboons were also collected to compare the initial immune activation level between STLV+ and STLV− animals. PBMCs were purified by Ficoll gradient centrifugation from heparin-anticoagulated peripheral venous blood.
Experimental protocol
| Treatment . | Monkey ID . | Age, y . | Sex . | Weight, kg . | Initial proviral load, copies/105 PBMCs . |
|---|---|---|---|---|---|
| None | C1 | 16 | Female | 19 | 519 |
| C2 | 20 | Male | 27 | 153 | |
| C3 | 11 | Male | 32 | 92 | |
| C4 | 15 | Female | 15 | 147 | |
| AZT | A1 | 14 | Female | 14 | 108 |
| A2 | 10 | Female | 22 | 86 | |
| VPA | V1 | 4 | Female | 10 | 292 |
| V2 | 5 | Female | 13 | 596 | |
| V3 | 5 | Female | 11 | 1092 | |
| V4 | 14 | Female | 18 | 2176 | |
| VPA + AZT | VA1 | 7 | Female | 19 | 2418 |
| VA2 | 5 | Female | 17 | 246 | |
| VA3 | 13 | Female | 20 | 441 | |
| VA4 | 12 | Female | 15 | 1164 | |
| VA5 | 4 | Male | 19 | 2660 |
| Treatment . | Monkey ID . | Age, y . | Sex . | Weight, kg . | Initial proviral load, copies/105 PBMCs . |
|---|---|---|---|---|---|
| None | C1 | 16 | Female | 19 | 519 |
| C2 | 20 | Male | 27 | 153 | |
| C3 | 11 | Male | 32 | 92 | |
| C4 | 15 | Female | 15 | 147 | |
| AZT | A1 | 14 | Female | 14 | 108 |
| A2 | 10 | Female | 22 | 86 | |
| VPA | V1 | 4 | Female | 10 | 292 |
| V2 | 5 | Female | 13 | 596 | |
| V3 | 5 | Female | 11 | 1092 | |
| V4 | 14 | Female | 18 | 2176 | |
| VPA + AZT | VA1 | 7 | Female | 19 | 2418 |
| VA2 | 5 | Female | 17 | 246 | |
| VA3 | 13 | Female | 20 | 441 | |
| VA4 | 12 | Female | 15 | 1164 | |
| VA5 | 4 | Male | 19 | 2660 |
Fifteen STLV-1–infected Papio papio were given daily a dose of VPA and/or AZT orally. The STLV-1 proviral load values are presented as the Tax copy number per 105 PBMCs at the initiation of the treatment. These values were also determined a month before the initiation of the trial and were found to be similar.
PBMCs indicates peripheral blood mononuclear cells; AZT, azidothymidine; VPA, valproate; and STLV-1, simian T-lymphotropic virus type 1.
STLV-1 PVL
PBMCs were resuspended in lysis buffer (10mM Tris-HCl, pH 8.0, 5mM ethylenediaminetetraacetic acid, 50mM NaCl, 0.5% sodium dodecyl sulfate, and 200 μg/mL proteinase K) and incubated overnight at 65°C. DNA was then purified by 3 consecutive extractions (phenol, phenol/chloroform, and chloroform). DNA was precipitated with isopropanol, washed with ethanol, and resuspended in PCR grade water. The STLV-1 and albumin specific primers and PCR protocol were previously described.36 Real-time PCR was carried out in a Roche light cycler using the DyNamo Capillary SYBR Green qPCR kit (Finnzymes) and the Light Cycler TaqMan master mix (Roche Diagnostics). Standard curves were generated using serial dilutions of DNA from the MT-4 cell line (which carries 7 proviral copies).
Flow cytometry
Lymphocytes isolated from heparin-anticoagulated blood were stained with the following monoclonal antibodies: anti-CD3–peridinin chlorophyll protein (clone SP34-2; BD Biosciences), anti-CD4–peridinin chlorophyll protein or –allophycocyanin (APC; clone L200, BD Biosciences), anti-CD8–phycoerythrin (PE) or –APC (clone RPA-T8; BD Biosciences), anti-CD20–fluorescein isothiocyanate (FITC; clone 2H7, BD Biosciences), anti-CD45RA–FITC (clone 5H9; BD Biosciences), anti-CD27–RPE (clone M-T271), anti-CD28–FITC or –PE (clone CD2.8, BD Biosciences), anti-CD95–PE or –APC (clone DX2, BD Biosciences), and anti-Ki67–FITC (clone MIB-1; Dako Denmark).
For cell surface staining, antibodies were added to the PBMCs for 1 hour at 4°C. PBMCs were then fixed and permeabilized with 250 μL of Cytofix/Cytoperm (BD Biosciences) for intracellular staining. After washing with Perm&Wash (BD Biosciences), the cells were incubated for 1 hour with anti-Ki67 antibodies. After washing, the cells were analyzed by flow cytometry as previously described.37 A total of 20 000 events in the lymphocyte gate were analyzed using FlowJo software (TreeStar). Absolute numbers of lymphocytes in blood were ascertained using routine diagnostic counts.
Lytic efficiency assay
The assay was performed with samples from 4 animals. The assay quantifies the rate of disappearance of CD4+ Tax-expressing cells caused by addition of autologous CD8+ cells.38 PBMCs were washed and the CD8+ cells positively selected with anti-CD8 (clone RPA-T8) coupled to magnetic microbeads (Miltenyi Biotec). The CD8+ cells were then titrated back into the CD8-depleted fraction from the same animal that had been isolated before treatment. Cells were then cocultivated at 37°C for 18 hours, harvested, and stained for Tax,39 CD4, and CD8 as described in the Introduction. The proportion of Tax+CD4+ cells surviving coculture was plotted against the proportion of CD8+ cells present. The lytic efficient rate (percentage CD4+Tax+ cells killed/percentage of CD8+ cells/day) was then calculated according to a previously described mathematical model.38 The assays were performed in duplicate, and the results are presented as the mean CD8+ cell lytic rate (“efficiency”).
Statistical analysis
Analyses were performed in GraphPad Prism software Version 5.0b. We used 1-tailed paired Student t tests to compare results in paired samples and Mann-Whitney tests to compare unpaired groups. P values less than .05 were considered significant. The nonlinear regression presented in Figure 5 was determined after a 1-phase exponential decay model.
Results
STLV-1 infection is associated with changes in T-lymphocyte populations
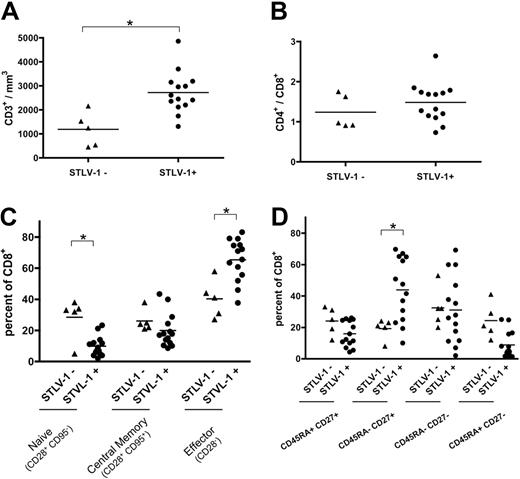
We initially compared the immunologic profiles of asymptomatic infected (STLV-1+) and uninfected (STLV-1−) baboons. Infected baboons had a higher total number of T lymphocytes (Mann-Whitney, P = .005; Figure 1A). However, the ratio between CD4+ and CD8+ T cells remained constant (Figure 1B). The viral infection also decreased the proportion of naive (CD28+CD95−) CD8+ T lymphocytes (Figure 1C, P = .02, Mann-Whitney) and increased the proportion of CD28− (Mann-Whitney, P = .008) and CD45RA- CD27+ (Mann-Whitney, P = .007) CD8+ effector T lymphocytes (Figure 1C-D). Similar differences in T-cell phenotypes were reported previously between uninfected humans and HAM/TSP patients.40 These results support the idea that the immune system of STLV-1–infected baboons is active but unable to clear the infection.
STLV-1 infection induces a constitutive activation of the simian immune system. (A) Comparison of the CD3+ cell number per cubed millimeter of blood between noninfected (5 animals) and STLV-1–infected (15 animals) monkeys before initiation of the treatment. Cells were stained with CD3 antibodies. The percentages obtained were then reported to the total lymphocyte number determined by clinical automated lymphocyte count. (B) Comparison of T-CD4+/T-CD8+ ratio between noninfected and infected monkeys before treatment. Cells were stained with CD4, CD8, and CD3 antibodies. (C) Comparison of CD8+ subpopulations count per cubed millimeter of blood determined before treatment. Cells were stained for CD3, CD8, CD95, and CD28. *Significant difference (P < .05; Mann-Whitney test).
STLV-1 infection induces a constitutive activation of the simian immune system. (A) Comparison of the CD3+ cell number per cubed millimeter of blood between noninfected (5 animals) and STLV-1–infected (15 animals) monkeys before initiation of the treatment. Cells were stained with CD3 antibodies. The percentages obtained were then reported to the total lymphocyte number determined by clinical automated lymphocyte count. (B) Comparison of T-CD4+/T-CD8+ ratio between noninfected and infected monkeys before treatment. Cells were stained with CD4, CD8, and CD3 antibodies. (C) Comparison of CD8+ subpopulations count per cubed millimeter of blood determined before treatment. Cells were stained for CD3, CD8, CD95, and CD28. *Significant difference (P < .05; Mann-Whitney test).
The combination of VPA and AZT reduced the PVL in asymptomatic animals
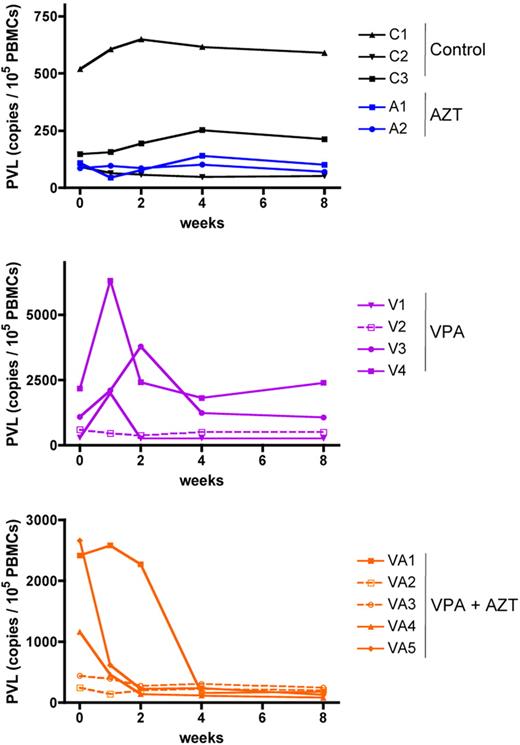
We then tested the effect of a treatment consisting of daily doses of VPA alone or in combination with AZT, given orally for 2 months to a series of male and female STLV-1–infected baboons whose PVL was quantified before the initiation of the trial (Table 1). In the absence of treatment, STLV-1 PVL did not change during observation for 2 months (paired Student t test, P = .27; Figure 2 top graph black lines). Consistent with previous HTLV-1 reports,9,41,42 AZT had no impact on the PVL after 2 months of treatment (paired Student t test, P = .11; Figure 2 top graph blue lines). As reported in HAM/TSP patients, VPA treatment induced a transient increase in the PVL during the first 2 weeks in 3 animals of 4. However, and contrary to what was previously reported,9,32 treatment with VPA alone did not cause a significant reduction of the PVL in STLV-1–infected baboons after 2 months of treatment (paired Student t test, P = .3; Figure 2, central panel, purple lines). Strikingly, the combined VPA/AZT treatment resulted in a 5- to 12-fold PVL reduction in the 3 animals with a PVL higher than 1 copy per 100 PBMCs. Crucially, the combined treatment prevented the transient increase in mean PVL that is seen after VPA alone (Figure 2 bottom graph orange lines).
Combined VPA/AZT treatment is linked to a significant reduction of the PVL in STLV-1 asymptomatic carriers. The PVL (copy number/105 PBMCs) was determined by real-time PCR, as previously described.36 For a given animal, all samples were run at the same time, to avoid interexperimental variation. The values presented here are the mean of 2 independent assays. C indicates controls; A, AZT; V, valproate; and VA, valproate + AZT.
Combined VPA/AZT treatment is linked to a significant reduction of the PVL in STLV-1 asymptomatic carriers. The PVL (copy number/105 PBMCs) was determined by real-time PCR, as previously described.36 For a given animal, all samples were run at the same time, to avoid interexperimental variation. The values presented here are the mean of 2 independent assays. C indicates controls; A, AZT; V, valproate; and VA, valproate + AZT.
Combined treatment with VPA and AZT resulted in an accumulation of CD8+ lymphocytes
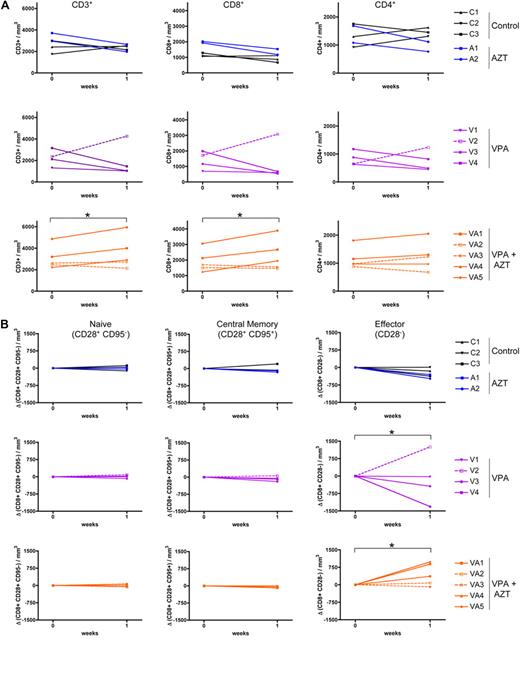
Because the increase in PVL occurred in animals treated with VPA alone during the first week of treatment, we focused our immunologic analyses on this period. There was no significant variation in the absolute number of T cells either in control (untreated) or AZT-treated animals (Figure 3A top row) during the first week of the trial (paired Student t test, P = .9 and P = .8, respectively). In all but 1 VPA-treated animal, the total T-cell number decreased, especially for CD8+ T cells (paired Student t test, P = .045; Figure 3A, central row). Interestingly, animal V2, which had no transient increase in PVL, also displayed an increased total lymphocyte number. Conversely, in the VPA-AZT-treated animals, the decrease in PVL was associated with a significant increase in the number of lymphocytes (paired Student t test, P = .016), principally of the CD8+ T-cell population (Figure 3A bottom row). In the 2 animals that did not display a major change in PVL after VPA/AZT treatment, the total lymphocyte count also remained approximately constant.
VPA/AZT treatment to an accumulation of CD8+ effector memory T lymphocytes. (A) Variation of CD3+, CD4+, and CD8+ cell number per cubed millimeter of blood was determined at days 0 and 7 of treatment. Cells were stained with CD4, CD8, and CD3 antibodies and analyzed by flow cytometry. The absolute numbers are derived from the absolute lymphocyte count previously determined by clinical automated systems. (B) Variation in the CD8+ subpopulations count was determined at days 0 and 7 of treatment. Cells were stained for CD3, CD8, CD95, and CD28. *Significant difference (P < .05; paired Student t test).
VPA/AZT treatment to an accumulation of CD8+ effector memory T lymphocytes. (A) Variation of CD3+, CD4+, and CD8+ cell number per cubed millimeter of blood was determined at days 0 and 7 of treatment. Cells were stained with CD4, CD8, and CD3 antibodies and analyzed by flow cytometry. The absolute numbers are derived from the absolute lymphocyte count previously determined by clinical automated systems. (B) Variation in the CD8+ subpopulations count was determined at days 0 and 7 of treatment. Cells were stained for CD3, CD8, CD95, and CD28. *Significant difference (P < .05; paired Student t test).
Combined VPA/AZT treatment results in an accumulation of CD8+ effector lymphocytes
To determine which T-cell subpopulations were altered during the treatment, naive, central memory, and effector CD8+ cell populations were analyzed 1-week after initiation of the treatment using 2 sets of markers: CD28/CD95 (Figure 3B) and CD45RA/CD27 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The numbers of CD28+CD95− (naive) and CD28+CD95+ (central memory) CD8+ T cells were unchanged in all 4 groups of animals (Kruskal-Wallis, P = .43 and .62, respectively). However, significant changes were observed in the CD8+ CD28− (effector) T-cell population (Figure 3B, right panels). The animals treated with VPA had a decrease in the number of CD8+ CD28− (effector) T cells (paired Student t test, P = .039), with the exception of the animal V2. In the VPA/AZT-treated animals, whose PVL decreased on treatment, the number of effector CD8+ CD28− T cells increased (paired Student t test, P = .039). This result was confirmed with the second set of differentiation markers: the CD8+ T-cell population whose number fluctuated was mainly CD45RA−CD27− (effector memory; paired Student t test, P = .02 and .036 for VPA and VPA/AZT, respectively; supplemental Figure 1). In contrast, the CD4+ T-cell count was not altered (supplemental Figure 2).
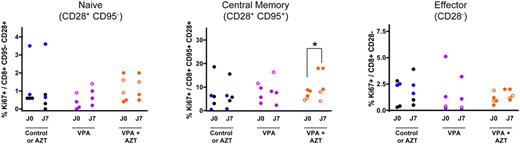
To determine the origin of the CD8+ population expansion, lymphocyte proliferation was quantified by analysis of Ki67 expression (Figure 4). The proportion of proliferating CD4+ cells was not modified in untreated, AZT-treated, or VPA-treated monkeys (data not shown), although a rebound in viral replication was observed in this latter group at 1 week of treatment. This observation strongly suggests that the transient PVL increase observed during VPA treatment is the consequence of new cycles of infection within the PBMCs. Conversely, the accumulation of effector CD8+ T lymphocytes (CD28−) in VPA/AZT-treated animals was associated with an increase in proliferation of the CD8+ central memory T-cell (CD28+CD95+) subset (paired Student t test, P = .035; Figure 4 central panel), consistent with the normal pathway of T-cell differentiation.
VPA/AZT treatment induces a proliferation of CD8+ central memory T lymphocytes. Variations in Ki67 expression (a marker for cell proliferation) was determined within the CD8+ T-cell subpopulations. The cells were stained for CD8, CD28, CD95, and Ki67. *Statistical difference (P < .05; paired Student t test).
VPA/AZT treatment induces a proliferation of CD8+ central memory T lymphocytes. Variations in Ki67 expression (a marker for cell proliferation) was determined within the CD8+ T-cell subpopulations. The cells were stained for CD8, CD28, CD95, and Ki67. *Statistical difference (P < .05; paired Student t test).
STLV-1 PVL reduction correlated with an accumulation of antiretroviral cytotoxic lymphocytes
We determined whether the accumulation of effector CD8+ T cells in VPA/AZT-treated monkeys represented an increase in the population of cytotoxic lymphocytes directed against STLV-1. To this end, we determined the rate of CD8+-mediated lysis of autologous, naturally STLV-1–infected cells at different time points (days 0, 7, and 15 of treatment). The results revealed a correlation between the variation in the CD8+ cell-mediated lysis rate and the variation in STLV-1 PVL (Figure 5).
STLV-1 PVL evolution is inversely correlated with the rate of CD8+ cell-mediated lysis of STLV-1–infected cells. The lytic efficiency rates (or ϵ) were determined at different times (at 0, 1, and 2 weeks of treatment) for the 4 baboons, whose Tax expression was detectable by flow cytometry in the PBMCs. The variations of the ϵ value over a time period of 1 week were plotted against the corresponding variations of PVL. The plot is overlaid with the line of best. The variations of PVL and ϵ correlate significantly according to a 1-phase exponential decay model (R2 = 0.63, or R2 = 0.989 if the outlier point is excluded).
STLV-1 PVL evolution is inversely correlated with the rate of CD8+ cell-mediated lysis of STLV-1–infected cells. The lytic efficiency rates (or ϵ) were determined at different times (at 0, 1, and 2 weeks of treatment) for the 4 baboons, whose Tax expression was detectable by flow cytometry in the PBMCs. The variations of the ϵ value over a time period of 1 week were plotted against the corresponding variations of PVL. The plot is overlaid with the line of best. The variations of PVL and ϵ correlate significantly according to a 1-phase exponential decay model (R2 = 0.63, or R2 = 0.989 if the outlier point is excluded).
Discussion
We analyzed the impact of a combination of VPA, an inhibitor of HDACi, and AZT, an inhibitor of the reverse transcriptase, in asymptomatic baboons naturally infected with STLV-1.
These nonhuman primates constitute the best model for studying HTLV-1–associated diseases and potential therapies because baboons are the natural hosts for STLV-1, a retrovirus closely related to HTLV-1. In simians, as in humans, the infection remains mainly asymptomatic. However, cases of ATLL-like retrovirus-associated diseases have been reported.35 We show here that the differences in the T-lymphocyte subpopulations associated with STLV-1 infection in baboons are also similar to those described in HTLV-1–infected humans.40 In each case, the retroviral infection is associated with an accumulation of effector T lymphocytes, which represent a marker of an immune response against the virus. One means of retroviral escape from the immune response is proviral silencing through epigenetic regulation. This is the reason why we hypothesized that drug-induced viral activation would promote viral clearance by the immune system.
A previous study described the use of VPA in HAM/TSP patients, which resulted in a significant decrease in the PVL after 45 to 60 days of treatment.9 We therefore hypothesized that VPA-based treatments could be given to ACs at risk of developing an HTLV-1–associated disease. Surprisingly, in our assay, administration of VPA alone did not cause any reduction in PVL during 2 months of treatment. However, and consistent with the previous report,9 we observed a transient but significant (up to a 3-fold increase) rise in PVL during the first week of treatment, suggesting that VPA treatment is not only unsuccessful but might be hazardous by increasing the rate at which HTLV-1–infected lymphocytes cross the blood-brain barrier, as suggested previously.32
In contrast, the VPA/AZT combination prevented the transient rise in PVL caused by VPA alone. More interestingly, it also induced a significant reduction in PVL in animals with a high initial PVL. The decrease in PVL was associated with an accumulation of effector CD8+ T lymphocytes and cytolytic lymphocytes directed against the virus. This strongly suggests that the observed PVL decrease was not the result of a direct apoptotic effect of the VPA on infected cells, as suggested by in vitro studies,30,31 but rather to an increase in the effectiveness of the anti–STLV-1 cellular immune response. The accumulation of cytotoxic lymphocytes is a consequence of an increased proliferation of memory CD8+ T cells. The proliferation of memory cells and subsequent accumulation of effector cells were not seen in the VPA group. We therefore suggest that the observed reduction in PVL was caused by an efficient immune response against the virus when de novo infection was prevented by addition of AZT. Our current working model is the following: in VPA/AZT-treated animals, infected cells express the virus in response to VPA treatment. When central memory lymphocytes recognize these cells, they proliferate, differentiate into effector cells, and kill STLV-1–infected cells, which leads to a decrease in PVL. On the contrary, in the absence of AZT, the virus spreads, and STLV-1-specific central memory cells become infected and are destroyed by autologous CD8+ T cells in a “fratricidal” response.13 Thus, the number of proliferating virus-specific memory cells decreases and cannot reconstitute the effector CD8+ reservoir. As a result, the PVL may increase.
As stated in the Introduction, the PVL is the strongest known correlate of inflammatory diseases in HTLV-1 infection. However, the only described therapy that reduces the PVL causes a large initial transient rise before the eventual fall.9 Our present data therefore represent the first indication that it is possible to make a substantial reduction in the PVL of an oncogenic retrovirus without a concomitant significant rise in the PVL shortly after starting treatment. This suggests that, when biomarkers are available, VPA/AZT could be given to HAM/TSP patients at the early stage of the disease. Finally, a recent nationwide prospective study conducted in Japan strongly suggested that a higher PVL is a strong factor in the development of ATLL.43 The VPA/AZT combination may therefore be used as a preventive treatment for other HTLV-1– related diseases such as ATLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sylviane Bassot for the serologic analyses and Emilie Coté for helpful suggestions. We also acknowledge the RsA-FD for their support.
This work was supported by La Ligue Nationale Contre le Cancer, l'Association de Recherche contre le Cancer (no. 1085), Cent pour sang la vie, and Inserm. R.M. was supported by Inserm and Inserm/AP-HP (contrat d'Interface vers l'hôpital) and is now supported by Ecole Normale Superieure de Lyon. F.M. and E.W. are supported by Inserm. P.V.A. was supported by the Ministère de la Recherche and the Pasteur-Weizmann Association.
Authorship
Contribution: P.V.A. and R.M. designed and performed research, analyzed data, and wrote the paper; M.M., F.M., F.T., and A.M. performed research; E.W., C.R.M.B., and Y.P. analyzed data; and A.G., J.E., O.H., and G.D. designed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renaud Mahieux, Inserm U758, Ecole Normale Supérieure de Lyon, 46 allée d'Italie, 69364 Lyon Cedex 07, France; e-mail: renaud.mahieux@ens-lyon.fr.
References
Author notes
O.H., J.E., and R.M. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal