Abstract
This study examines the prognostic significance of early molecular response using an expanded dataset in chronic myeloid leukemia patients enrolled in the International Randomized Study of Interferon and STI571 (IRIS). Serial molecular studies demonstrate decreases in BCR-ABL transcripts over time. Analyses of event-free survival (EFS) and time to progression to accelerated phase/blast crisis (AP/BC) at 7 years were based on molecular responses using the international scale (IS) at 6-, 12-, and 18-month landmarks. Patients with BCR-ABL transcripts > 10% at 6 months and > 1% at 12 months had inferior EFS and higher rate of progression to AP/BC compared with all other molecular response groups. Conversely, patients who achieved major molecular response [MMR: BCR-ABL (IS) ≤ 0.1%] by 18 months enjoyed remarkably durable responses, with no progression to AP/BC and 95% EFS at 7 years. The probability of loss of complete cytogenetic response by 7 years was only 3% for patients in MMR at 18 months versus 26% for patients with complete cytogenetic response but not MMR (P < .001). This study shows a strong association between the degree to which BCR-ABL transcript numbers are reduced by therapy and long-term clinical outcome, supporting the use of time-dependent molecular measures to determine optimal response to therapy. This study is registered at www.clinicaltrials.gov as NCT00006343.
Introduction
The International Randomized Study of Interferon and STI571 (IRIS) trial demonstrated the dramatic effectiveness of the tyrosine kinase inhibitor imatinib in newly diagnosed chronic phase chronic myeloid leukemia (CML-CP). At 18 months, the rate of complete cytogenetic response (CCyR) in patients treated with imatinib was 76% versus 15% (P < .001) for patients treated with interferon (IFN) plus cytarabine.1 For a complete list of IRIS participants, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). At 7 years of follow-up, patients randomized to the imatinib arm of IRIS who remain on imatinib therapy continue to show durable hematologic and cytogenetic responses, low progression rates to accelerated phase or blast crisis (AP/BC), and excellent survival outcomes.1-4 Seven-year results from the IRIS trial demonstrated an overall survival (OS) rate of 86% for patients randomized to imatinib, exceeding reported survival rates for all previous CML therapies.4 The rates of relapse and progression are low for patients treated with imatinib, with an overall estimated event-free survival (EFS) rate of 81% and transformation-free survival (to AP/BC) of 93% at 7 years. Among patients randomized to imatinib, 82% achieved a CCyR, of whom 17% had documented loss of CCyR during treatment.
The IRIS trial was the first randomized trial to demonstrate the prognostic significance of monitoring BCR-ABL transcripts by real-time quantitative polymerase chain reaction (RQ-PCR). The first report of the molecular monitoring from IRIS was based on 18-month median follow-up data.5 It showed that patients in the imatinib group who had a reduction in the level of BCR-ABL transcripts of > 3-log (n = 137) compared with a standardized baseline had a negligible risk of disease progression over the subsequent 12 months. Because of this prognostic association, a > 3-log reduction in BCR-ABL was defined as a major molecular response (MMR).6 Among patients who had achieved a CCyR, 100% of those who had achieved an MMR survived without progression to AP/BC at 24 months compared with 95% for patients who had not achieved a similar 3-log reduction in BCR-ABL transcript levels.6 Subsequently, Druker and colleagues confirmed that the achievement of an MMR continued to be associated with an improved outcome at 60 months, with estimated rates without progression to AP/BC of 100%, 98%, and 87% for patients achieving CCyR and MMR, CCyR without MMR, and no CCyR, respectively.2
At the outset of the IRIS trial, MMR was considered an exploratory end point, and per protocol was only studied in patients who had obtained a CCyR. As planned, molecular monitoring in the IRIS trial ended at 24 months and thus was not routinely performed in months 24 to 48. Routine molecular analysis was reinstated every 6 months from month 48 onward, and in addition, a project to standardize molecular diagnostic laboratories on a global level was initiated so that BCR-ABL transcript levels could be standardized and reported on an internationally agreed scale.7
Previous reports of IRIS trial results presented molecular data collected from only patients on first-line imatinib who had achieved a CCyR and who had molecular samples collected per IRIS protocol.2 However, additional PCR samples were also collected from first-line imatinib patients participating in preplanned substudies of molecular responses, as well as ad hoc samples collected from other patients. This paper presents the first analysis of the complete molecular monitoring dataset and demonstrates the long-term prognostic value of the levels of molecular response at specific time points.
Methods
Patients and samples
Patients enrolled on the imatinib arm of the IRIS trial with at least 1 BCR-ABL transcript measurement were included in these analyses. Samples for RQ-PCR were collected as follows: (1) after achievement of CCyR; (2) at regular intervals as part of preplanned substudies conducted at German, Australian, and New Zealand sites independent of cytogenetic response status; and (3) at physicians' discretion before the achievement of CCyR. Patients in the German substudy had molecular analyses conducted at 1, 2, and 3 months and then every 3 months thereafter.8 Patients in Australia and New Zealand had molecular assessments performed at 3-month intervals, in addition to the per-protocol assessments. The IRIS study was conducted in accordance with the Declaration of Helsinki and applicable regulatory requirements. The protocol was approved by the institutional review board or ethics committee of each participating center. All patients gave written informed consent before participation.
Determination of the level of response and standardization of assay across laboratories
Statistical analysis
All analyses presented in this document were based on patients with at least 1 PCR assessment who were treated with first-line imatinib treatment. Rates of molecular response categories over time were obtained based on the available PCR assessments at each time point. In addition, summary statistics (mean, medians, and quartiles) of BCR-ABL ratios at each time point were calculated. The data were graphically displayed, and the Kaplan-Meier estimated rates summarized with 95% confidence intervals (CIs). Statistical tests for all time-to-event analyses were based on the log-rank test.
The long-term clinical outcomes were analyzed based on the molecular response categories at landmark time points (6, 12, and 18 months). For these landmark analyses, patients had to have received first-line imatinib from the onset of treatment up to, or beyond, the specified time point. Patients were categorized according to their molecular response level. Patients who achieved their “end point event” before a given landmark time point were excluded from the corresponding analysis and from subsequent landmark analyses. For example, any patient with an EFS event before 12 months was excluded from the 12-month landmark analysis, or any patient who progressed to AP/BC before 18 months was excluded from the 18-month landmark. The following long-term outcomes were considered: (1) EFS, which is defined as the time from treatment start until any of the following events that occur during study treatment: (i) loss of complete hematologic response (CHR), (ii) loss of major cytogenetic response (MCyR), (iii) progression to AP/BC, or (iv) death due to any cause; (2) time to AP/BC or CML-related death; (3) time to loss of CCyR in patients who achieved a CCyR at or before the landmark; (4) OS; and (5) the slope of falling tendency of BCR-ABL ratio during the last 3 years, estimated using linear regression methods and modeled as optimal fit, representing the whole patient population of 116 patients who had PCR measurements at month 84. In an additional analysis, loss of CCyR was included as an event for EFS to match current clinical practice definitions of EFS. Generally, EFS and time to AP/BC were censored at the last assessment date (date of hematologic or cytogenetic evaluations) for patients who did not progress. Censoring date for OS data was the date of last contact.
Results
Samples
The total IRIS PCR dataset consisted of 3627 blood samples from 476 of 553 patients enrolled on the imatinib arm of the IRIS trial. This included 98 patients as part of substudies who had PCR assessments independent of cytogenetic response status. The median follow-up from start of treatment until last PCR sample was 77 months for the 476 patients who had at least 1 PCR sample collected.
Patient characteristics
To ensure that the inclusion of data from patients in the various substudies did not bias the analysis, a comparison of the substudy and nonsubstudy patients was performed. No differences were observed between the intention-to-treat (ITT) population (n = 553), those with PCR data (n = 476), and patients in the substudy populations (n = 98) with regard to demographics, disposition, dose intensity, time on treatment, and length of follow-up (Table 1).
Demographics and disease characteristics at baseline
| Variable . | Substudy population* (n = 98) . | PCR population† (n = 476) . | ITT population (n = 553) . |
|---|---|---|---|
| Age, y | |||
| Mean | 48.2 | 48.0 | 48.2 |
| SD | 13.2 | 12.6 | 12.6 |
| Median | 50 | 51 | 50 |
| Q1, Q3 | 39, 59 | 39, 58 | 39, 58 |
| Range | 20-69 | 18-70 | 18-70 |
| Sex, n (%) | |||
| Male | 65 (66.3) | 296 (62.2) | 342 (61.8) |
| Female | 33 (33.7) | 180 (37.8) | 211 (38.2) |
| Race, n (%) | |||
| White | 92 (93.9) | 425 (89.3) | 494 (89.3) |
| Black | 5 (5.1) | 23 (4.8) | 28 (5.1) |
| Other | 1 (1.0) | 28 (5.9) | 31 (5.6) |
| Weight, kg | |||
| n | 95 | 467 | 540 |
| Mean | 78 | 80.4 | 80.4 |
| SD | 15.9 | 17.9 | 18.2 |
| Median | 76 | 78.5 | 78.7 |
| Q1, Q3 | 68, 87.9 | 68, 89.6 | 68, 89.5 |
| Range | 48-134 | 42.5-169.5 | 40.0-169.5 |
| ECOG performance status, n (%) | |||
| Missing data | 1 (1.0) | 4 (0.8) | 5 (0.9) |
| 0 | 76 (77.6) | 368 (77.3) | 425 (76.9) |
| 1 | 18 (18.4) | 96 (20.2) | 115 (20.8) |
| 2 | 3 (3.1) | 8 (1.7) | 8 (1.4) |
| Sokal risk group, n (%) | |||
| Low | 33 (33.7) | 175 (36.8) | 201 (36.3) |
| Intermediate | 23 (23.5) | 99 (20.8) | 111 (20.1) |
| High | 12 (12.2) | 63 (13.2) | 71 (12.8) |
| Not known | 30 (30.6) | 139 (29.2) | 170 (30.7) |
| Variable . | Substudy population* (n = 98) . | PCR population† (n = 476) . | ITT population (n = 553) . |
|---|---|---|---|
| Age, y | |||
| Mean | 48.2 | 48.0 | 48.2 |
| SD | 13.2 | 12.6 | 12.6 |
| Median | 50 | 51 | 50 |
| Q1, Q3 | 39, 59 | 39, 58 | 39, 58 |
| Range | 20-69 | 18-70 | 18-70 |
| Sex, n (%) | |||
| Male | 65 (66.3) | 296 (62.2) | 342 (61.8) |
| Female | 33 (33.7) | 180 (37.8) | 211 (38.2) |
| Race, n (%) | |||
| White | 92 (93.9) | 425 (89.3) | 494 (89.3) |
| Black | 5 (5.1) | 23 (4.8) | 28 (5.1) |
| Other | 1 (1.0) | 28 (5.9) | 31 (5.6) |
| Weight, kg | |||
| n | 95 | 467 | 540 |
| Mean | 78 | 80.4 | 80.4 |
| SD | 15.9 | 17.9 | 18.2 |
| Median | 76 | 78.5 | 78.7 |
| Q1, Q3 | 68, 87.9 | 68, 89.6 | 68, 89.5 |
| Range | 48-134 | 42.5-169.5 | 40.0-169.5 |
| ECOG performance status, n (%) | |||
| Missing data | 1 (1.0) | 4 (0.8) | 5 (0.9) |
| 0 | 76 (77.6) | 368 (77.3) | 425 (76.9) |
| 1 | 18 (18.4) | 96 (20.2) | 115 (20.8) |
| 2 | 3 (3.1) | 8 (1.7) | 8 (1.4) |
| Sokal risk group, n (%) | |||
| Low | 33 (33.7) | 175 (36.8) | 201 (36.3) |
| Intermediate | 23 (23.5) | 99 (20.8) | 111 (20.1) |
| High | 12 (12.2) | 63 (13.2) | 71 (12.8) |
| Not known | 30 (30.6) | 139 (29.2) | 170 (30.7) |
ECOG indicates Eastern Cooperative Oncology Group; ITT, intention to treat; PCR, polymerase chain reaction; Q1, quartile 1; Q3, quartile 3; and SD, standard deviation.
Substudy population; patients with PCR samples collected at regular intervals as part of preplanned substudies conducted at German, Australian, and New Zealand sites independent of cytogenetic response status.
PCR population; patients with at least 1 PCR sample, including patients from the substudy and nonsubstudy populations.
Major molecular response rates over time
The rates of molecular response improved over time in both substudy and nonsubstudy patients in a similar fashion (Figure 1). Please note that the 36 months' time point does not include any “nonsubstudy patients.” According to the original protocol, samples were only to be collected up to 24 months, and PCR sampling was later reintroduced by a study amendment. The overall trend in BCR-ABL levels from month 48 to 84 indicates an ongoing reduction in transcript levels (a decrease of 0.37512-log units). During this period, a 58% reduction in the BCR-ABL ratio was observed at 84 months compared with the BCR-ABL ratio value at 48 months. Overall, at 84 months, rates of MMR were 87% and 92% (in patients with available samples), and the median BCR-ABL ratio (IS) was 0.003% and 0.004% for nonsubstudy and substudy patients, respectively.
Median BCR-ABL (IS) transcript levels in substudy and nonsubstudy patients over 84 months. Lines along x-axis connect medians over time; vertical bars represent 25th and 75th percentiles; vertical lines represent range.
Median BCR-ABL (IS) transcript levels in substudy and nonsubstudy patients over 84 months. Lines along x-axis connect medians over time; vertical bars represent 25th and 75th percentiles; vertical lines represent range.
Among the 98 patients treated in Australia, New Zealand, and Germany (substudy population), 24 (24%) had achieved an MMR at 6 months and 38 (39%) at 12 months. The 12-month MMR rate in this PCR substudy corresponds to the estimated rate published in 2003, in which the rate of MMR in CCyR patients (57%) was multiplied with the CCyR rate by 12 months (68%) to estimate the MMR rate in the overall population at 12 months (39%).1 In the IRIS substudy population, the MMR rate increased to 64 (65%) of all 98 patients (ITT population) at 5 years (90% based on number of patients with available samples). It should be noted that the 98 patients treated in the substudy population is the ITT population, in which patients without samples were considered as nonresponders. Therefore, the number of patients with PCR samples at each individual time point is always less than 98 (ie, never exceeds 86). To avoid a potential selection bias resulting from using only available samples, the MMR rates were also determined based on the total number of substudy patients (n = 98). As a consequence, the “real” rate of MMR at 5 years is somewhere between 65% (ITT based on 98 patients) and 90% (based on available samples with varying number of patients). The best observed MMR rate in these 98 patients was 86% with current follow-up.
CCyR and molecular response
The association of molecular response and CCyR from the 3-month to 18-month landmarks is shown in Table 2. At 3 months, 75% of patients with a CCyR had already achieved a BCR-ABL (IS) ≤ 1.0%, and the rate of CCyR increased to 95% of patients with BCR-ABL (IS) ≤ 1.0% by the 12-month landmark. The percentage of MMR in patients who were in CCyR at the time the MMR was tested increased from 33% at 3 months to 78% at 18 months. Conversely, at 3 months, 89% of patients who had achieved an MMR also had evidence of a CCyR, whereas at 18 months, 96% of those with an MMR had a CCyR. At 18 months, 6 patients with MMR and cytogenetic assessment did not have a CCyR. However, 5 patients had only 1 Phildelphia chromosome-positive (Ph+) of 20-31 metaphases and 1 patient had 2 Ph+ of 40 metaphases evaluated. Of these 6 patients, 4 had achieved CCyR before 18 months and again after, 1 achieved CCyR only after 18 months, and 1 patient only had documented PCyR during study. None of these 6 patients has progressed or died.
Molecular response levels in patients with CCyR
| Time point, mo . | Number of patients with CCyR and PCR assessments . | BCR-ABL ratio (IS) categories (2 best categories) in patients with CCyR, % . | Total ≤ 1.0% in patients with CCyR, % . | |
|---|---|---|---|---|
| n . | ≤ 0.1% (MMR) . | > 0.1% to ≤ 1.0% . | ≤ 1% . | |
| 3 | 51 | 33.3 | 41.2 | 74.5 |
| 6 | 127 | 48.0 | 41.7 | 89.7 |
| 9 | 138 | 47.1 | 39.9 | 87.0 |
| 12 | 177 | 62.1 | 32.8 | 94.9 |
| 18 | 163 | 77.9 | 16.6 | 94.5 |
| Time point, mo . | Number of patients with CCyR and PCR assessments . | BCR-ABL ratio (IS) categories (2 best categories) in patients with CCyR, % . | Total ≤ 1.0% in patients with CCyR, % . | |
|---|---|---|---|---|
| n . | ≤ 0.1% (MMR) . | > 0.1% to ≤ 1.0% . | ≤ 1% . | |
| 3 | 51 | 33.3 | 41.2 | 74.5 |
| 6 | 127 | 48.0 | 41.7 | 89.7 |
| 9 | 138 | 47.1 | 39.9 | 87.0 |
| 12 | 177 | 62.1 | 32.8 | 94.9 |
| 18 | 163 | 77.9 | 16.6 | 94.5 |
CCyR indicates complete cytogenetic response; MMR, major molecular response; and PCR, polymerase chain reaction.
Landmark analyses
To determine whether BCR-ABL (IS) values at 6, 12, and 18 months were predictive of long-term EFS, time to AP/BC, and OS, the rates for EFS, AP/BC, and OS were evaluated by molecular response at 3 landmarks.
EFS by molecular response at 6, 12, and 18 months
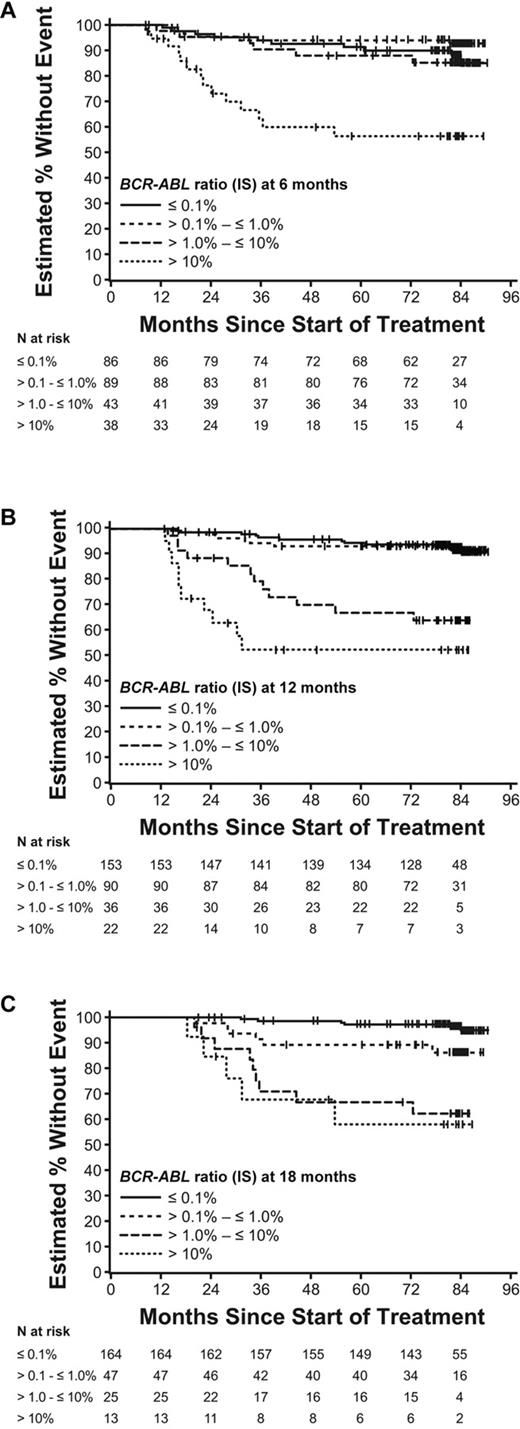
Categories of molecular response were associated with EFS in a time-dependent manner. At the 6-month landmark, patients with a poor molecular response had lower EFS rates compared with patients who achieved better molecular responses. Thus, patients with a BCR-ABL (IS) > 10% had 84-month EFS rates that were reduced (56%) compared with patients with a BCR-ABL (IS) ≤ 0.1%, BCR-ABL (IS) > 0.1% to ≤ 1.0%, and those with BCR-ABL (IS) > 1% to ≤ 10%, all of whom had an EFS rate > 85% at 84 months (Table 3 and Figure 2A). At 12 months, the achievement of an MMR appeared to be a clinically significant event. The EFS in patients with an MMR [BCR-ABL (IS) ≤ 0.1%] at 12 months was 91%, compared with 79% in cases who had not achieved an MMR (P = .001; Table 3 and Figure 2B). At the 12-month landmark, patients with an MMR, and those patients with a BCR-ABL (IS) level at the category below > 0.1% to ≤ 1.0%, had similar 84-month EFS rates; however, those with a worse molecular response fared more poorly, with EFS of < 65%. Lastly, after 18 months, the significance of achieving an MMR became more pronounced compared with achieving lesser levels of response. Thus, the EFS for patients with an MMR was 95%, compared with 86% in patients achieving the next lower category of response of IS > 0.1% to ≤ 1.0% (P = .01; Table 3 and Figure 2C). Patients with even poorer molecular response [BCR-ABL (IS) > 1.0%] suffered a worse EFS of < 65%. In the IRIS study, loss of CCyR was not considered as an event. However, because current clinical practice considers it to represent imatinib failure,14 EFS analyses were also performed taking losses of CCyR into account (Table 4). When loss of CCyR was considered as an additional event, EFS rates for patients with an MMR, BCR-ABL (IS) > 0.1% to ≤ 1.0%, and BCR-ABL (IS) > 1.0% to ≤ 1.0%, were 84%, 81%, and 78%, respectively, at 6 months; 87%, 84%, and 57%, respectively, at 12 months; and 92%, 79%, and 48%, respectively, at 18 months. The majority of samples in both categories [MMR and BCR-ABL (IS) > 0.1% to ≤ 1.0%] were from patients who had achieved CCyR and thus had samples collected according to protocol.
Long-term outcomes (estimated rates at 7 years with 95% CIs) by molecular response level at 6, 12, and 18 months (landmark analyses)
| Landmark, % (95% CI) . | BCR-ABL ratio (IS) categories . | Log-rank P . | |||||
|---|---|---|---|---|---|---|---|
| MMR . | No MMR . | Total no MMR . | |||||
| ≤ 0.1% . | > 0.1 to ≤ 1.0% . | > 1.0 to ≤ 10% . | > 10% . | > 0.1% . | Comparing MMR vs no MMR . | Comparing MMR vs > 0.1 to ≤ 1% . | |
| 6 mo | n = 86 | n = 89 | n = 44 | n = 39 | n = 172 | ||
| EFS rate, % | 85.1 (76; 94) | 92.8 (87; 98) | 85.2 (74; 96) | 56.3 (39; 74) | 83.5 (78; 89) | ns | ns |
| Without AP/BC | 96.2 (92; 100) | 98.4 (95; 100) | 95.2 (89; 100) | 75.8 (60; 92) | 93 (89; 97) | ns | ns |
| OS rate | 90.3 (83; 97) | 93.0 (88; 98) | 100 (100; 100) | 68.2 (53; 83) | 89 (85; 94) | ns | ns |
| 12 mo | n = 153 | n = 90 | n = 36 | n = 25 | n = 151 | ||
| EFS rate | 91 (85; 97) | 91.7 (86; 98) | 64.1 (48; 80) | 52.5 (31; 74) | 79.4 (73; 86) | .001† | ns‡ |
| Without AP/BC | 99 (97; 100)* | 95.5 (91; 100) | 83.4 (70; 97) | 76 (57; 95) | 89.9 (85; 95) | .0004† | .048‡ |
| OS rate | 92.5 (88; 97) | 96.7 (93; 100) | 85.7 (74; 97) | 65.5 (46; 85) | 89.2 (84; 94) | ns | ns |
| 18 mo | n = 164 | n = 48 | n = 25 | n = 16 | n = 89 | ||
| EFS rate | 94.9 (91; 99) | 86.4 (76; 97) | 62.3 (43; 82) | 58.0 (30; 87) | 75.3 (66; 85) | < .001† | .014‡ |
| Without AP/BC | 99.1 (98; 100)* | 95.7 (90; 100) | 82.6 (67; 98) | 81.5 (58; 100) | 90.1 (84; 97) | < .001† | .054 |
| OS rate | 94.9 (91; 99) | 95.7 (90; 100) | 84.0 (70; 98) | 80.8 (61; 100) | 89.8 (84; 96) | ns | ns |
| Landmark, % (95% CI) . | BCR-ABL ratio (IS) categories . | Log-rank P . | |||||
|---|---|---|---|---|---|---|---|
| MMR . | No MMR . | Total no MMR . | |||||
| ≤ 0.1% . | > 0.1 to ≤ 1.0% . | > 1.0 to ≤ 10% . | > 10% . | > 0.1% . | Comparing MMR vs no MMR . | Comparing MMR vs > 0.1 to ≤ 1% . | |
| 6 mo | n = 86 | n = 89 | n = 44 | n = 39 | n = 172 | ||
| EFS rate, % | 85.1 (76; 94) | 92.8 (87; 98) | 85.2 (74; 96) | 56.3 (39; 74) | 83.5 (78; 89) | ns | ns |
| Without AP/BC | 96.2 (92; 100) | 98.4 (95; 100) | 95.2 (89; 100) | 75.8 (60; 92) | 93 (89; 97) | ns | ns |
| OS rate | 90.3 (83; 97) | 93.0 (88; 98) | 100 (100; 100) | 68.2 (53; 83) | 89 (85; 94) | ns | ns |
| 12 mo | n = 153 | n = 90 | n = 36 | n = 25 | n = 151 | ||
| EFS rate | 91 (85; 97) | 91.7 (86; 98) | 64.1 (48; 80) | 52.5 (31; 74) | 79.4 (73; 86) | .001† | ns‡ |
| Without AP/BC | 99 (97; 100)* | 95.5 (91; 100) | 83.4 (70; 97) | 76 (57; 95) | 89.9 (85; 95) | .0004† | .048‡ |
| OS rate | 92.5 (88; 97) | 96.7 (93; 100) | 85.7 (74; 97) | 65.5 (46; 85) | 89.2 (84; 94) | ns | ns |
| 18 mo | n = 164 | n = 48 | n = 25 | n = 16 | n = 89 | ||
| EFS rate | 94.9 (91; 99) | 86.4 (76; 97) | 62.3 (43; 82) | 58.0 (30; 87) | 75.3 (66; 85) | < .001† | .014‡ |
| Without AP/BC | 99.1 (98; 100)* | 95.7 (90; 100) | 82.6 (67; 98) | 81.5 (58; 100) | 90.1 (84; 97) | < .001† | .054 |
| OS rate | 94.9 (91; 99) | 95.7 (90; 100) | 84.0 (70; 98) | 80.8 (61; 100) | 89.8 (84; 96) | ns | ns |
Patients with event (or censored) before the landmark are excluded from the landmark analyses (ie, 2, 3, and 4 patients in the “No MMR” category are excluded for analyses of EFS after the 6, 12, and 18 months' landmark, respectively; for AP/BC, 2, 1, 1 patients, respectively; for OS, 1 patient was censored before 12 months at the 12-month landmark).
AP/BC indicates accelerated phase or blast crisis; CI, confidence interval; EFS, event-free survival; MMR, major molecular response; ns, not significant; and OS, overall survival.
EFS = loss of CHR, loss of MCyR, progression to AP/BC, death due to any cause on treatment. Progression to AP/BC = progression to AP/BC, CML-related deaths on treatment.
This reflects an event that was recorded as progression between months 72 and 84. The records of this patient were subsequently reviewed and the event was a death not due to CML. This data correction could not be reflected in the database after cutoff and database lock. Therefore, the estimated rate without progression to AP/BC was 100% in patients who had achieved MMR at 12 or 18 months.
Log-rank test MMR versus no MMR. The log-rank test calculates a P value testing the null hypothesis that the 2 survival functions are the same (ie, that the long-term outcome is similar between the responder groups).
Log-rank test (MMR versus > 0.1 to ≤ 1.0%).
EFS at 6 (A), 12 (B), and 18-month (C) landmarks by molecular response. These figures use the original EFS definition, without loss of CCyR counting as an event.
EFS at 6 (A), 12 (B), and 18-month (C) landmarks by molecular response. These figures use the original EFS definition, without loss of CCyR counting as an event.
Event-free survival (estimated rates at 7 years with 95% CIs), including loss of CCyR as an event by molecular response level at 6, 12, and 18 months (landmark analyses)
| Landmark . | BCR-ABL ratio (IS) categories . | Log-rank P . | |||||
|---|---|---|---|---|---|---|---|
| MMR . | No MMR . | Total no MMR . | |||||
| ≤ 0.1% . | > 0.1 to ≤ 1.0% . | > 1.0 to ≤ 10% . | > 10% . | ≥ 0.1% . | Comparing MMR vs no MMR . | Comparing MMR vs > 0.1 to ≤ 1% . | |
| 6 mo | n = 86 | n = 89 | n = 43 | n = 38 | n = 170 | ||
| EFS rate, % (95% CI) | 84.4 (75; 94) | 81.1 (72; 90) | 78.0 (65; 91) | 36.5 (19; 54) | 71.6 (64; 79) | .0114* | ns† |
| 12 mo | n = 151 | n = 89 | n = 33 | n = 20 | n = 142 | ||
| EFS rate, % (95% CI) | 86.6 (80; 94) | 84.0 (76; 92) | 57.2 (39; 75) | 48.5 (26; 71) | 73.1 (65; 81) | .0006* | ns† |
| 18 mo | n = 160 | n = 43 | n = 22 | n = 13 | n = 78 | ||
| EFS rate, % (95% CI) | 92.3 (87; 98) | 78.5 (66; 91) | 47.7 (26; 70) | 48.0 (18; 78) | 65.4 (54; 77) | < .001* | .0019† |
| Landmark . | BCR-ABL ratio (IS) categories . | Log-rank P . | |||||
|---|---|---|---|---|---|---|---|
| MMR . | No MMR . | Total no MMR . | |||||
| ≤ 0.1% . | > 0.1 to ≤ 1.0% . | > 1.0 to ≤ 10% . | > 10% . | ≥ 0.1% . | Comparing MMR vs no MMR . | Comparing MMR vs > 0.1 to ≤ 1% . | |
| 6 mo | n = 86 | n = 89 | n = 43 | n = 38 | n = 170 | ||
| EFS rate, % (95% CI) | 84.4 (75; 94) | 81.1 (72; 90) | 78.0 (65; 91) | 36.5 (19; 54) | 71.6 (64; 79) | .0114* | ns† |
| 12 mo | n = 151 | n = 89 | n = 33 | n = 20 | n = 142 | ||
| EFS rate, % (95% CI) | 86.6 (80; 94) | 84.0 (76; 92) | 57.2 (39; 75) | 48.5 (26; 71) | 73.1 (65; 81) | .0006* | ns† |
| 18 mo | n = 160 | n = 43 | n = 22 | n = 13 | n = 78 | ||
| EFS rate, % (95% CI) | 92.3 (87; 98) | 78.5 (66; 91) | 47.7 (26; 70) | 48.0 (18; 78) | 65.4 (54; 77) | < .001* | .0019† |
AP/BC indicates accelerated phase or blast crisis; CHR, complete hematologic response; CI, confidence interval; CCyR, complete cytogenetic response; EFS, event-free survival; IS, international scale; MCyR, major cytogenetic response; MMR, major molecular response; and ns, not significant.
Patients with event (including loss of CCyR) before the landmark are excluded from the landmark analyses. EFS (loss of CHR, loss of MCyR, progression to AP/BC, death due to any cause on treatment) or loss of CCyR.
Log-rank test MMR versus no MMR.
Log-rank test (MMR versus > 0.1 to ≤ 1.0%). The log-rank test calculates a P value testing the null hypothesis that the 2 survival functions are the same (ie, that the long-term outcome is similar between the responder groups).
Based on the 6-month landmark, loss of CCyR accounted for 1 of 11 events in the MMR patients and 10 of 16 events in the second response category. In the 12-month landmark, loss of CCyR accounted for 7 of 17 events in the MMR category and 7 of 14 in the second response category. For the 18-month landmark, loss of CCyR accounted for 4 of 10 events in the MMR patients and 3 of 9 events in the second response category. The remaining events were either (CML-unrelated) death on treatment, loss of MCyR (mostly in CCyR patients; ie, an increase in Ph+ metaphases to > 35% and not only > 0%). The rate of loss of CCyR is very similar to the rate of loss reported by the Hammersmith group15 for patients who were in CCyR but not in MMR at 18 months.
The strongest relationship of MMR and durability of CCyR was at the 18-month landmark, in which there was a statistically significant difference in the maintenance of CCyR between patients in MMR and those with a molecular response > 0.1% to ≤ 1.0%. Whereas an estimated 97% of patients with MMR at 18 months remained in CCyR at 84 months, only 74% of the patients with molecular response > 0.1% to ≤ 1% remained in CCyR at that time (P < .001; Figure 3).
Progression to AP/BC by molecular response at 6, 12, and 18 months
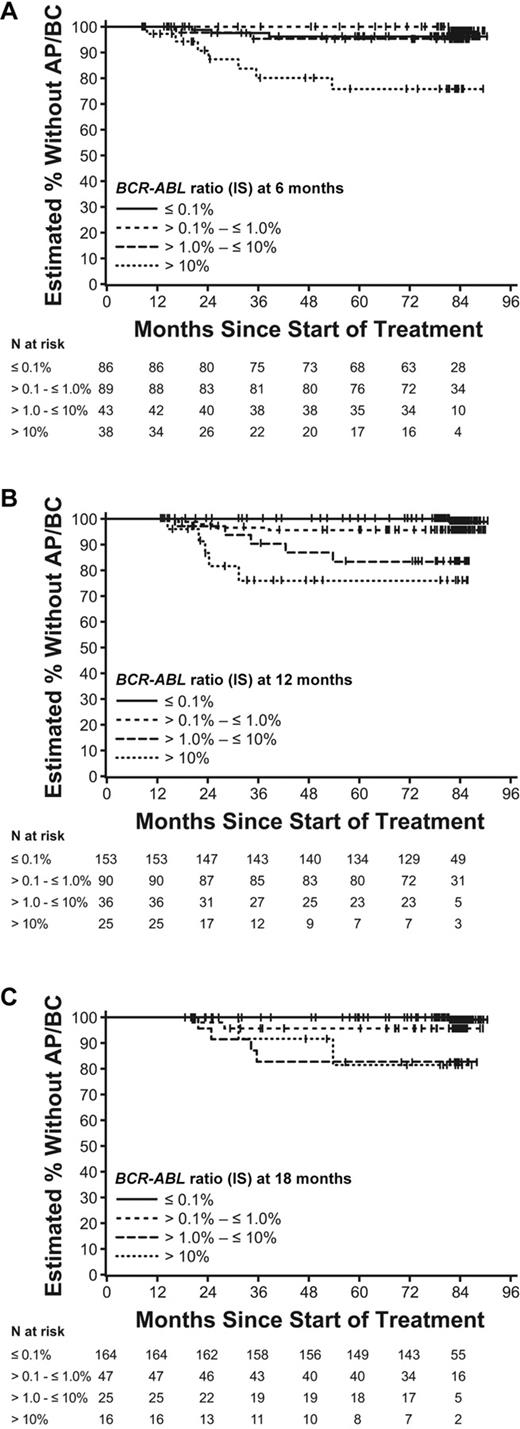
Achievement of MMR at 12 months was associated with decreased rate of progression to AP/BC (99% progression-free survival for patients with an MMR vs 90% for patients without an MMR; P = .0004; Table 3 and Figure 4A-C). In addition, patients who achieved an MMR had lower progression rates than those who had achieved a response at the next lowest category of response, that of BCR-ABL (IS) > 0.1% to ≤ 1.0% (99% vs 96%, P = .048; Table 3 and Figure 4C). The association of MMR versus lack of MMR and progression-free rate was also seen at the 18-month landmark (99% vs 90%, respectively; P < .001). Of note, the only patient with an MMR at 12 and 18 months who had been considered as having progressed to AP/BC had died due to unknown reasons. This event was confirmed to be unrelated to CML with the subsequent data update, so none of the patients with documented MMR at 12 or 18 months has progressed on imatinib study treatment.
Time to AP/BC according to 6 (A), 12 (B), and 18-month (C) landmarks by molecular response.
Time to AP/BC according to 6 (A), 12 (B), and 18-month (C) landmarks by molecular response.
Overall survival by molecular response at 6, 12, and 18 months
At 6 months, molecular response did not distinguish patients' favorable and unfavorable outcomes, except for those patients with the poorest molecular response. Thus, the OS for patients with a 6-month molecular response of an IS > 10% was only 68%, compared with > 90% in all other categories of molecular response. The same relationship with molecular response and OS held at the 12-month landmark. At the 18-month landmark, there was a trend for improved OS in patients who obtained a molecular response of IS ≤ 1.0%, with OS of ≈ 95% compared with 84% in cases with an IS of > 1.0% to ≤ 10% (Table 3).
Discussion
With more than 7 years of follow-up, BCR-ABL transcript levels continued to decrease in responding patients who remained on imatinib in the IRIS trial, suggesting further reduction in the size of the leukemia progenitor pool over the long term. BCR-ABL transcript levels at 6, 12, and 18 months were predictive of long-term EFS (originally defined without considering loss of CCyR as an event) and freedom from progression to AP/BC. MMR at 12 months predicted for remarkably high rates of EFS at 84 months. It is important to note that the achievement of BCR-ABL values below 1.0% and greater than 0.1% at 12 months was associated with equally favorable rates of EFS compared with values below 0.1%. However, whereas 5 of the 11 events in the MMR group were CML-unrelated deaths and none was progression to AP/BC, 4 of the 7 events in the category below 1.0% BCR-ABL were progressions to AP/BC and included 1 CML-related death. At 18 months, MMR was associated with improved EFS over even a 2- to 3-log reduction in BCR-ABL (BCR-ABL [IS] > 0.1% to ≤ 1.0%). The lack of association between attainment of MMR and OS may have been due to the fact that patients failing to achieve milestones were able to switch to second-generation agents that also provide high OS rates for patients resistant to imatinib therapy. Perhaps the most persuasive evidence of the prognostic importance of achieving MMR by 12 and 18 months is that none of these patients has progressed to AP/BC to date. These data suggest that attaining an MMR may be a “safe haven” that promises optimal long-term outcomes in CML patients.
These data confirm that CCyR can be estimated accurately with the BCR-ABL mRNA level in peripheral blood. An earlier study showed that CCyR was reached at a BCR-ABL/ABL level of 2%16 ; in the present study, the level associated with CCyR was 1.0% on the IS, consistent with the previous trial. Seventy-five percent of patients who achieved CCyR at 3 months had also achieved a BCR-ABL (IS) ≤ 1.0%; this increased to 95% of patients by 18 months. In addition, achievement of an MMR at any time predicted for maintenance of CCyR at 84 months. These findings suggest that it may be possible to use molecular monitoring in place of cytogenetic monitoring once CCyR is attained. These data are consistent with the analysis of more than 800 peripheral blood PCR samples and simultaneous bone marrow cytogenetic samples by Ross et al, which showed that, after 6 months on imatinib, the probability of cytogenetic progression without a significant increase in BCR-ABL transcript level is extremely low; thus, PCR can be used to determine the need for any further cytogenetic assessment.17 Based on the lack of progression events observed once patients achieved an MMR, these data support discontinuing cytogenetic assessments after the achievement of MMR and using molecular monitoring to determine patient response. If MMR is lost, then regular cytogenetic analysis should be resumed. A possible objection to this policy is based on the observation that some patients in continuing CCyR acquire new cytogenetic abnormalities in the Philadelphia-negative clone; the new clones are usually “benign,” but in a minority they are associated with features suggesting myelodysplastic syndromes, including deteriorating peripheral blood counts. Therefore, if a patient in continuing molecular response has an unexplained fall in counts, cytogenetic studies could be indicated to rule out a secondary hematologic disorder.
CML has been the model for demonstrating the power of molecular testing in defining response and predicting outcomes.18-24 Before the introduction of imatinib, testing for BCR-ABL following stem cell transplantation identified patients at high risk of relapse, and protocols designed to abort relapse by adding donor leukocyte infusion and IFN (and recently, tyrosine kinase inhibitors) have evolved in many centers.25-29 In addition, defining persistent molecular remissions by RQ-PCR has been important in managing IFN and transplant patients to define cases as “cured.”16,30 Similarly, defining patients in a “complete molecular remission (CMR),” ie, those patients with persistently undetectable BCR-ABL (> 2 years),31 may prove important clinically, as several ongoing studies have suggested that some of these patients may be able to discontinue imatinib therapy without subsequent relapse.32-34 However, it should be noted that > 50% of these patients who discontinued imatinib therapy did not maintain CMR, and discontinuation of tyrosine kinase inhibitor therapy in responding patients is not recommended outside of a clinical trial setting. Nonetheless, CMR and MMR may evolve into important end points for clinical research and routine patient management in CML, as well as other hematologic malignancies.
In summary, the routine monitoring of BCR-ABL transcripts, in conjunction with cytogenetic evaluation, offers clinicians and patients important information about the long-term prospects of disease control in CML. It is likely that the lessons learned in CML will translate to the clinical management of other types of leukemias.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Daniel Hutta, PhD, and Erinn Goldman, PhD, for medical editorial assistance with this manuscript. We thank Florence Combel for programming all of the outputs.
This work was supported by a Practitioner Fellowship from the National Health and Medical Research Council of Australia (T.P.H.), Deutsche José Carreras-Leukämiestiftung (DJCLS H03/01; A.H.); and NIH/NCI CA 18 029 (J.P.R.). Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Authorship
Contribution: T.P.H., A.H., M.S.R., M.M., and J.P.R. designed and performed research; S.B., J.K., M.C.M., L.F., B.J.D., F.G., R.A.L., S.G.O'B., and J.M.G. performed research; T.P.H., A.H., S.B., J.K., M.C.M., L.F., B.J.D., R.A.L., S.G.O'B., M.S.R., M.M., E.W., V.M., J.M.G., and J.P.R. collected, analyzed, and interpreted data; E.W. performed statistical analysis; and all authors drafted and approved the manuscript.
Conflict-of-interest disclosure: T.P.H. has received research funding and honoraria from Novartis and Bristol-Myers Squibb; A.H. has received research funding and provided consultancy for Novartis; S.B. has received research funding and honoraria and provided consultancy for Novartis; M.C.M. and J.K. have no relevant financial relationships to disclose; L.F. has received research funding (PhD studentship) from Novartis; B.J.D. has provided consultancy for Ambit Biosciences, Avalon Pharmaceuticals, Calistoga Pharmaceuticals, Cylene Pharmaceuticals, and Roche, has equity ownership with MolecularMD, has received research funding from Novartis, ARIAD, Bristol-Myers Squibb, and has patents and royalties from OHSU patent no. 843 Mutate ABL Kinase Domains, and Millipore via Dana-Farber Cancer Institute; F.G. has received research funding and honoraria from Novartis and Bristol-Myers Squibb; R.A.L. has received research funding and provided consultancy for Novartis; S.G.O. has provided consultancy for Bristol-Myers Squibb, received research funding from Novartis, Bristol-Myers Squibb, and Wyeth, and received honoraria from Bristol-Myers Squibb; M.S.R., M.M., E.W., and V.M. are Novartis employees and stockholders; J.M.G. has received honoraria and participated on speakers' bureaux for Novartis and Bristol-Myers Squibb; and J.P.R. has received research funding and honoraria and provided consultancy for Novartis.
Correspondence: Timothy P. Hughes, Department of Haematology, SA Pathology, Royal Adelaide Hospital, Frome Rd, Adelaide, 5000, Australia; e-mail: timothy.hughes@health.sa.gov.au.
References
Author notes
T.H. and A.H. contributed equally to this work.