Abstract
Changing definitions and classifications of hematologic malignancies (HMs) complicate incidence comparisons. HAEMACARE classified HMs into groupings consistent with the latest World Health Organization classification and useful for epidemiologic and public health purposes. We present crude, age-specific and age-standardized incidence rates for European HMs according to these groupings, estimated from 66 371 lymphoid malignancies (LMs) and 21 796 myeloid malignancies (MMs) registered in 2000-2002 by 44 European cancer registries, grouped into 5 regions. Age-standardized incidence rates were 24.5 (per 100 000) for LMs and 7.55 for MMs. The commonest LMs were plasma cell neoplasms (4.62), small B-cell lymphocytic lymphoma/chronic lymphatic leukemia (3.79), diffuse B-cell lymphoma (3.13), and Hodgkin lymphoma (2.41). The commonest MMs were acute myeloid leukemia (2.96), other myeloproliferative neoplasms (1.76), and myelodysplastic syndrome (1.24). Unknown morphology LMs were commonest in Northern Europe (7.53); unknown morphology MMs were commonest in Southern Europe (0.73). Overall incidence was lowest in Eastern Europe and lower in women than in men. For most LMs, incidence was highest in Southern Europe; for MMs incidence was highest in the United Kingdom and Ireland. Differences in diagnostic and registration criteria are an important cause of incidence variation; however, different distribution of HM risk factors also contributes. The quality of population-based HM data needs further improvement.
Introduction
Hematologic malignancies (HMs) are a heterogeneous group of diseases of diverse incidence, prognosis, and etiology. Most population-based studies on the incidence of HMs have grouped these diseases into broad categories: Hodgkin versus non-Hodgkin lymphoma, acute versus chronic, and lymphatic versus myeloid leukemia.1,2
Comparison of HM incidence across regions and over time is complicated by the existence of different disease classification systems and by the fact that the criteria for disease definition vary between countries, and even between treatment centers and cancer registries (CRs) within a country.3 The situation is further complicated by the major changes in HM classification that have occurred in recent years. The most recent HM classifications, the third revision of the International Classification of Diseases-Oncology (ICD-O-3) published in 20004 and the closely related World Health Organization (WHO) publications5,6 classify HMs at the most basic level according to cell lineage and cell maturity but use morphologic, genotypic, genetic, and immunohistochemical criteria, as well as clinical behavior, to further subdivide these entities. The ICD-O-3 classification is thought to have been applied retrospectively by most European CRs to their HM incident data from the year 2000.
HAEMACARE is a European CR-based project funded by the European Commission and set up in 2005 to improve the standardization and availability of population-based data on HMs archived by EUROCARE CRs.7,8 Under the aegis of HAEMACARE, hematologists, pathologists, and epidemiologists from several European countries reached a consensus on the grouping of lymphoid and myeloid neoplasms (as defined by ICD-O-3 morphology codes and WHO recommendations) into categories based primarily on cell lineage but with subcategories based on similar prognosis and therefore useful for epidemiologic and public health purposes. The HAEMACARE grouping system thus produced incorporates the latest changes introduced by the WHO classification6 and is consistent with the classification of lymphoid neoplasms for epidemiologic research proposed by the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) in 2007.9
The aim of this study is to present and analyze data on HM incidence from European CRs, classified according to ICD-O-3 morphology codes, and grouped according to HAEMACARE indications. To ensure analysis of a relatively homogeneous set of cases, only cases incident from 2000-2002 were considered.
Methods
Cancer registries
The present EUROCARE network includes most, but not all, European CRs and covers approximately 30% of the European population. All EUROCARE CRs were invited to participate in the present HAEMACARE study, but only 48 CRs, operating in 20 countries,8 had incidence data for at least one of the predefined study years (2000-2002). Eleven of the CRs participating in the present study cover populations of entire countries; the other CRs cover variable percentages of national populations. The CRs were grouped into 5 geographic regions: Northern Europe (Iceland, Norway, and Sweden); United Kingdom and Ireland (England, Ireland, Northern Ireland, Scotland, and Wales); Central Europe (Austria, France, Germany, Switzerland, and The Netherlands); Southern Europe (Italy, Malta, Slovenia, and Spain); and Eastern Europe (Czech Republic, Poland, and Slovakia).
The proportion of national coverage and number of cases contributed by each CR, with age at diagnosis, are shown in Table 1, together with indicators of data quality. Thirty-nine CRs provided incidence data for 2000-2002, 5 for 2000-2001, and 4 for 2000, for a total of 97 521 incident cases.
Percentage of national coverage, coverage period, reference population, number of cases, mean and median age at diagnosis, and data quality indicators for 48 European CRs with incidence data in 2000-2002 for hematologic malignancies
| European region/country . | Cancer registry . | National coverage,* percentage . | Cases diagnosed in 2000-2002 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years covered . | Average population per year . | Total cases . | Mean age, y . | Median age, y . | Death certificate only/autopsy, percentage . | Verified microscopically, percentage . | Unknown morphology,† percentage . | |||
| Northern Europe | 16 837 | 65 | 70 | 1.5 | 99.0 | 25.3 | ||||
| Iceland | Iceland | 100.0 | 2000-2002 | 282 995 | 243 | 61 | 67 | 0.4 | 100.0 | 0.8 |
| Norway | Norway | 100.0 | 2000-2002 | 4 502 000 | 5229 | 65 | 69 | 1.0 | 97.0 | 16.6 |
| Sweden | Sweden | 100.0 | 2000-2002 | 8 884 449 | 11 365 | 66 | 70 | 1.7 | 99.9 | 29.8 |
| United Kingdom and Ireland | 39 575 | 65 | 69 | 2.9 | 90.2 | 15.2 | ||||
| Ireland | Ireland | 100.0 | 2000-2002 | 3 836 871 | 4303 | 62 | 67 | 2.8 | 95.7 | 21.0 |
| England | United Kingdom East Anglia | 5.4 | 2000-2002 | 2 749 513 | 3972 | 66 | 70 | 1.4 | 95.1 | 16.2 |
| United Kingdom Northern and Yorkshire | 13.3 | 2000-2002 | 6 563 034 | 8962 | 66 | 70 | 1.1 | 95.8 | 14.0 | |
| United Kingdom Oxford | 5.4 | 2000-2002 | 2 732 200 | 2897 | 63 | 67 | 0.5 | 100.0 | 16.1 | |
| United Kingdom West Midlands | 10.7 | 2000-2002 | 5 284 832 | 6281 | 65 | 69 | 6.4 | 84.0 | 12.5 | |
| Northern Ireland | United Kingdom Northern Ireland | 100.0 | 2000-2002 | 1 689 635 | 1694 | 63 | 67 | 1.3 | 76.1 | 29.7 |
| Scotland | United Kingdom Scotland | 100.0 | 2000-2002 | 5 060 647 | 7623 | 66 | 70 | 0.5 | 95.5 | 11.1 |
| Wales | United Kingdom Wales | 100.0 | 2000-2002 | 2 913 498 | 3843 | 66 | 70 | 10.8 | 64.3 | 15.9 |
| Central Europe | 16 587 | 63 | 68 | 5.2 | 93.2 | 17.0 | ||||
| Austria‡ | Austria | 100.0 | 2000-2002 | 8 029 426 | 6998 | 64 | 69 | 10.3 | 86.8 | 32.3 |
| France | Cote d'Or (Cancer Registry of Haematological Malignancies) | 0.9 | 2000-2002 | 508 222 | 741 | 66 | 71 | 0.0 | 100.0 | 3.5 |
| Germany | Saarland | 1.3 | 2000-2002 | 1 067 443 | 1269 | 64 | 67 | 4.5 | 91.0 | 7.1 |
| Switzerland | Basel | 6.1 | 2000-2001 | 433 027 | 278 | 64 | 68 | 3.2 | 99.6 | 5.8 |
| Geneva | 5.6 | 2000-2002 | 417 096 | 532 | 64 | 68 | 0.6 | 98.1 | 17.3 | |
| St Gallen | 7.2 | 2000-2002 | 519 583 | 621 | 63 | 68 | 1.3 | 100.0 | 6.3 | |
| Ticino | 4.3 | 2000-2002 | 311 549 | 413 | 63 | 67 | 2.7 | 94.9 | 6.5 | |
| The Netherlands | Amsterdam | 17.6 | 2000-2002 | 2 872 613 | 2685 | 61 | 65 | 0.6 | 99.1 | 2.9 |
| Eindhoven | 6.1 | 2000-2001 | 989 680 | 581 | 60 | 64 | 0.0 | 97.9 | 8.5 | |
| North Netherlands | 12.9 | 2000-2001 | 2 070 146 | 1380 | 62 | 66 | 2.3 | 98.5 | 5.4 | |
| Twente | 7.2 | 2000-2002 | 1 156 162 | 1089 | 62 | 67 | 0.7 | 99.0 | 5.6 | |
| Southern Europe | 17 972 | 64 | 68 | 1.1 | 92.4 | 17.4 | ||||
| Italy | Alto Adige | 0.8 | 2000-2002 | 465 938 | 504 | 64 | 68 | 0.0 | 99.0 | 5.0 |
| Biella | 0.3 | 2000-2002 | 187 983 | 370 | 66 | 70 | 0.3 | 95.7 | 3.2 | |
| Ferrara | 0.6 | 2000-2002 | 347 156 | 611 | 67 | 71 | 0.8 | 98.7 | 7.9 | |
| Firenze | 2.0 | 2000-2002 | 1 162 973 | 1941 | 64 | 68 | 0.6 | 69.0 | 21.5 | |
| Friuli Venezia Giulia | 2.1 | 2000-2002 | 1 192 711 | 1918 | 65 | 69 | 1.6 | 99.6 | 29.1 | |
| Genova | 1.6 | 2000 | 900 735 | 579 | 67 | 70 | 0.9 | 79.3 | 7.8 | |
| Modena | 1.1 | 2000-2002 | 638 743 | 1045 | 63 | 68 | 0.3 | 99.4 | 2.6 | |
| Napoli | 0.9 | 2000 | 546 399 | 192 | 51 | 57 | 1.0 | 76.6 | 23.6 | |
| Parma | 0.7 | 2000-2002 | 402 788 | 769 | 66 | 70 | 0.0 | 100.0 | 15.9 | |
| Ragusa | 0.5 | 2000-2002 | 295 496 | 453 | 64 | 69 | 1.6 | 95.4 | 4.9 | |
| Reggio Emilia‡ | 0.8 | 2000-2002 | 462 469 | 734 | 65 | 69 | 0.1 | 97.3 | 50.7 | |
| Romagna | 1.7 | 2000-2002 | 991 045 | 1713 | 66 | 70 | 2.3 | 97.5 | 11.7 | |
| Salerno | 1.9 | 2000-2001 | 1 082 710 | 700 | 61 | 66 | 2.7 | 93.4 | 22.7 | |
| Sassari | 0.8 | 2000-2002 | 469 200 | 576 | 62 | 66 | 0.3 | 96.7 | 5.6 | |
| Torino | 1.6 | 2000-2001 | 900 408 | 887 | 64 | 67 | 1.0 | 95.4 | 22.0 | |
| Trento | 0.8 | 2000 | 477 859 | 200 | 66 | 70 | 0.5 | 96.5 | 9.0 | |
| Umbria‡ | 1.5 | 2000-2002 | 833 506 | 1125 | 64 | 69 | 0.7 | 77.6 | 38.8 | |
| Veneto | 3.5 | 2000 | 2 015 290 | 944 | 64 | 68 | 1.9 | 92.6 | 14.5 | |
| Malta | Malta | 100.0 | 2000-2002 | 388 752 | 378 | 60 | 65 | 0.0 | 98.4 | 17.5 |
| Slovenia | Slovenia | 100.0 | 2000-2002 | 1 990 625 | 1652 | 60 | 66 | 0.9 | 100.0 | 8.8 |
| Spain | Girona | 1.3 | 2000-2002 | 558 649 | 681 | 64 | 70 | 3.4 | 95.5 | 5.7 |
| Eastern Europe | 6550 | 60 | 65 | 9.8 | 91.4 | 14.5 | ||||
| Czech Republic | West Bohemia | 8.3 | 2000-2002 | 854 583 | 839 | 61 | 65 | 7.0 | 91.9 | 12.8 |
| Poland | Cracow‡ | 1.9 | 2000-2002 | 731 162 | 497 | 60 | 65 | 8.5 | 82.9 | 34.2 |
| Kielce | 3.1 | 2000-2002 | 1 325 260 | 972 | 60 | 66 | 0.0 | 89.2 | 23.5 | |
| Warsaw | 4.2 | 2000-2002 | 1 673 830 | 1130 | 61 | 66 | 0.0 | 97.2 | 13.6 | |
| Slovakia | Slovakia | 100.0 | 2000-2002 | 5 385 464 | 3112 | 58 | 64 | 17.3 | 91.2 | 9.3 |
| Total | 97 521 | 64 | 69 | 3.2 | 92.7 | 17.6 | ||||
| European region/country . | Cancer registry . | National coverage,* percentage . | Cases diagnosed in 2000-2002 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years covered . | Average population per year . | Total cases . | Mean age, y . | Median age, y . | Death certificate only/autopsy, percentage . | Verified microscopically, percentage . | Unknown morphology,† percentage . | |||
| Northern Europe | 16 837 | 65 | 70 | 1.5 | 99.0 | 25.3 | ||||
| Iceland | Iceland | 100.0 | 2000-2002 | 282 995 | 243 | 61 | 67 | 0.4 | 100.0 | 0.8 |
| Norway | Norway | 100.0 | 2000-2002 | 4 502 000 | 5229 | 65 | 69 | 1.0 | 97.0 | 16.6 |
| Sweden | Sweden | 100.0 | 2000-2002 | 8 884 449 | 11 365 | 66 | 70 | 1.7 | 99.9 | 29.8 |
| United Kingdom and Ireland | 39 575 | 65 | 69 | 2.9 | 90.2 | 15.2 | ||||
| Ireland | Ireland | 100.0 | 2000-2002 | 3 836 871 | 4303 | 62 | 67 | 2.8 | 95.7 | 21.0 |
| England | United Kingdom East Anglia | 5.4 | 2000-2002 | 2 749 513 | 3972 | 66 | 70 | 1.4 | 95.1 | 16.2 |
| United Kingdom Northern and Yorkshire | 13.3 | 2000-2002 | 6 563 034 | 8962 | 66 | 70 | 1.1 | 95.8 | 14.0 | |
| United Kingdom Oxford | 5.4 | 2000-2002 | 2 732 200 | 2897 | 63 | 67 | 0.5 | 100.0 | 16.1 | |
| United Kingdom West Midlands | 10.7 | 2000-2002 | 5 284 832 | 6281 | 65 | 69 | 6.4 | 84.0 | 12.5 | |
| Northern Ireland | United Kingdom Northern Ireland | 100.0 | 2000-2002 | 1 689 635 | 1694 | 63 | 67 | 1.3 | 76.1 | 29.7 |
| Scotland | United Kingdom Scotland | 100.0 | 2000-2002 | 5 060 647 | 7623 | 66 | 70 | 0.5 | 95.5 | 11.1 |
| Wales | United Kingdom Wales | 100.0 | 2000-2002 | 2 913 498 | 3843 | 66 | 70 | 10.8 | 64.3 | 15.9 |
| Central Europe | 16 587 | 63 | 68 | 5.2 | 93.2 | 17.0 | ||||
| Austria‡ | Austria | 100.0 | 2000-2002 | 8 029 426 | 6998 | 64 | 69 | 10.3 | 86.8 | 32.3 |
| France | Cote d'Or (Cancer Registry of Haematological Malignancies) | 0.9 | 2000-2002 | 508 222 | 741 | 66 | 71 | 0.0 | 100.0 | 3.5 |
| Germany | Saarland | 1.3 | 2000-2002 | 1 067 443 | 1269 | 64 | 67 | 4.5 | 91.0 | 7.1 |
| Switzerland | Basel | 6.1 | 2000-2001 | 433 027 | 278 | 64 | 68 | 3.2 | 99.6 | 5.8 |
| Geneva | 5.6 | 2000-2002 | 417 096 | 532 | 64 | 68 | 0.6 | 98.1 | 17.3 | |
| St Gallen | 7.2 | 2000-2002 | 519 583 | 621 | 63 | 68 | 1.3 | 100.0 | 6.3 | |
| Ticino | 4.3 | 2000-2002 | 311 549 | 413 | 63 | 67 | 2.7 | 94.9 | 6.5 | |
| The Netherlands | Amsterdam | 17.6 | 2000-2002 | 2 872 613 | 2685 | 61 | 65 | 0.6 | 99.1 | 2.9 |
| Eindhoven | 6.1 | 2000-2001 | 989 680 | 581 | 60 | 64 | 0.0 | 97.9 | 8.5 | |
| North Netherlands | 12.9 | 2000-2001 | 2 070 146 | 1380 | 62 | 66 | 2.3 | 98.5 | 5.4 | |
| Twente | 7.2 | 2000-2002 | 1 156 162 | 1089 | 62 | 67 | 0.7 | 99.0 | 5.6 | |
| Southern Europe | 17 972 | 64 | 68 | 1.1 | 92.4 | 17.4 | ||||
| Italy | Alto Adige | 0.8 | 2000-2002 | 465 938 | 504 | 64 | 68 | 0.0 | 99.0 | 5.0 |
| Biella | 0.3 | 2000-2002 | 187 983 | 370 | 66 | 70 | 0.3 | 95.7 | 3.2 | |
| Ferrara | 0.6 | 2000-2002 | 347 156 | 611 | 67 | 71 | 0.8 | 98.7 | 7.9 | |
| Firenze | 2.0 | 2000-2002 | 1 162 973 | 1941 | 64 | 68 | 0.6 | 69.0 | 21.5 | |
| Friuli Venezia Giulia | 2.1 | 2000-2002 | 1 192 711 | 1918 | 65 | 69 | 1.6 | 99.6 | 29.1 | |
| Genova | 1.6 | 2000 | 900 735 | 579 | 67 | 70 | 0.9 | 79.3 | 7.8 | |
| Modena | 1.1 | 2000-2002 | 638 743 | 1045 | 63 | 68 | 0.3 | 99.4 | 2.6 | |
| Napoli | 0.9 | 2000 | 546 399 | 192 | 51 | 57 | 1.0 | 76.6 | 23.6 | |
| Parma | 0.7 | 2000-2002 | 402 788 | 769 | 66 | 70 | 0.0 | 100.0 | 15.9 | |
| Ragusa | 0.5 | 2000-2002 | 295 496 | 453 | 64 | 69 | 1.6 | 95.4 | 4.9 | |
| Reggio Emilia‡ | 0.8 | 2000-2002 | 462 469 | 734 | 65 | 69 | 0.1 | 97.3 | 50.7 | |
| Romagna | 1.7 | 2000-2002 | 991 045 | 1713 | 66 | 70 | 2.3 | 97.5 | 11.7 | |
| Salerno | 1.9 | 2000-2001 | 1 082 710 | 700 | 61 | 66 | 2.7 | 93.4 | 22.7 | |
| Sassari | 0.8 | 2000-2002 | 469 200 | 576 | 62 | 66 | 0.3 | 96.7 | 5.6 | |
| Torino | 1.6 | 2000-2001 | 900 408 | 887 | 64 | 67 | 1.0 | 95.4 | 22.0 | |
| Trento | 0.8 | 2000 | 477 859 | 200 | 66 | 70 | 0.5 | 96.5 | 9.0 | |
| Umbria‡ | 1.5 | 2000-2002 | 833 506 | 1125 | 64 | 69 | 0.7 | 77.6 | 38.8 | |
| Veneto | 3.5 | 2000 | 2 015 290 | 944 | 64 | 68 | 1.9 | 92.6 | 14.5 | |
| Malta | Malta | 100.0 | 2000-2002 | 388 752 | 378 | 60 | 65 | 0.0 | 98.4 | 17.5 |
| Slovenia | Slovenia | 100.0 | 2000-2002 | 1 990 625 | 1652 | 60 | 66 | 0.9 | 100.0 | 8.8 |
| Spain | Girona | 1.3 | 2000-2002 | 558 649 | 681 | 64 | 70 | 3.4 | 95.5 | 5.7 |
| Eastern Europe | 6550 | 60 | 65 | 9.8 | 91.4 | 14.5 | ||||
| Czech Republic | West Bohemia | 8.3 | 2000-2002 | 854 583 | 839 | 61 | 65 | 7.0 | 91.9 | 12.8 |
| Poland | Cracow‡ | 1.9 | 2000-2002 | 731 162 | 497 | 60 | 65 | 8.5 | 82.9 | 34.2 |
| Kielce | 3.1 | 2000-2002 | 1 325 260 | 972 | 60 | 66 | 0.0 | 89.2 | 23.5 | |
| Warsaw | 4.2 | 2000-2002 | 1 673 830 | 1130 | 61 | 66 | 0.0 | 97.2 | 13.6 | |
| Slovakia | Slovakia | 100.0 | 2000-2002 | 5 385 464 | 3112 | 58 | 64 | 17.3 | 91.2 | 9.3 |
| Total | 97 521 | 64 | 69 | 3.2 | 92.7 | 17.6 | ||||
Proportion of national population covered by each registry in 1995-1999.
Unknown morphology (NOS) includes the following ICD-O-3 codes: 9590, 9591, 9800, 9801, 9805, 9820, 9832, and 9860.
CR was excluded from the final analysis because NOS cases exceeded 30%.
To obtain a set of cases with adequately specified morphology, we excluded CRs for which not otherwise specified (NOS) morphology constituted ≥ 30% of cases. The ICD-O-3 NOS codes are: lymphoma, 9590; non-Hodgkin lymphoma (NHL), 9591; lymphatic leukemia, 9820; leukemia, 9832; acute leukemia, 9800 and 9801; ambiguous lineage, 9805; and myeloid leukemia, 9860. The CRs of Austria, Cracow (Poland), Reggio Emilia (Italy), and Umbria (Italy) were excluded for this reason. The resulting study population, from the remaining 44 CRs, consisted of 88 167 cases: 66 371 lymphoid and 21 796 myeloid.
Data completeness
The present dataset was collected principally for the purposes of survival analysis. To investigate incidence completeness, the age-standardized incidence rates for Hodgkin lymphoma (HL), immunoproliferative disease, multiple myeloma, and myeloid leukemia, in the present dataset were compared with the incidence rates published in volume IX of Cancer Incidence in 5 Continents (CI5),1 for the same CRs over the same incidence period. CI5 is the official publication of population-based CRs worldwide and can be considered the “gold standard” because only data from CRs satisfying CI5's stringent criteria for data quality and completeness are published. The results of the comparison (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) showed, in all cases, that age-standardized incidence rates were closely similar, indicating that our data were as complete as those of CI5.
HM categories
ICD-O-3 codes for HMs were grouped into the 2 main disease lineages (lymphoid and myeloid) according to WHO indications. In accord with the HAEMACARE7 and InterLymph9 recommendations, lymphoid malignancies were grouped into 5 major categories (Table 2): HL, mature B-cell neoplasms, mature T-cell and natural killer cell neoplasms (T-NK), lymphoblastic lymphoma/acute (precursor cell) lymphatic leukemia (LL/ALL), and lymphoid NOS. These groups were subdivided according to lineage, again in accord with WHO and HAEMACARE (Table 2). Specifically, small B-cell lymphocytic lymphoma (SBLL)/chronic lymphatic leukemia (CLL) were analyzed together, as were Burkitt lymphoma and Burkitt leukemia (as noted previously). LL/ALL were divided into B-cell, T-cell, and NOS types. Within the mature B-cell neoplasm category, mature B-cell leukemia includes prolymphocytic leukemia B-cell type and mature hairy cell B leukemia, and plasma cell neoplasms were a major subcategory.
Number of cases and crude incidence rates (IR) per 100 000 for lymphoid malignancies by sex and morphologic type diagnosed in 2000-2002 and archived in 44 European CRs
| HAEMACARE groupings . | ICD-O-3 code . | ICD-O-3 description . | No. of cases . | All . | Males . | Females . | |||
|---|---|---|---|---|---|---|---|---|---|
| IR . | 95% CI . | IR . | 95% CI . | IR . | 95% CI . | ||||
| HL | 5571 | 2.49 | (2.42-2.55) | 2.81 | (2.71-2.91) | 2.18 | (2.09-2.26) | ||
| HL, nodular lymphocyte predominance | 9659 | HL, nodular lymphocyte predominance | 195 | 0.09 | (0.08-0.10) | 0.12 | (0.10-0.15) | 0.05 | (0.04-0.07) |
| Classic HL | 5376 | 2.40 | (2.34-2.47) | 2.69 | (2.59-2.79) | 2.12 | (2.04-2.21) | ||
| 9650 | HL, NOS | 996 | 0.44 | (0.42-0.47) | 0.52 | (0.48-0.57) | 0.37 | (0.34-0.41) | |
| 9661 | Hodgkin granuloma (obsolete) | ||||||||
| 9662 | Hodgkin sarcoma (obsolete) | ||||||||
| 9651 | HL, lymphocyte rich | 217 | 0.10 | (0.08-0.11) | 0.13 | (0.11-0.15) | 0.07 | (0.05-0.08) | |
| 9663 | HL, nodular sclerosis, NOS | 3165 | 1.41 | (1.36-1.46) | 1.45 | (1.38-1.53) | 1.37 | (1.31-1.44) | |
| 9664 | HL, nodular sclerosis cellular phase | ||||||||
| 9665 | HL, nodular sclerosis grade 1 | ||||||||
| 9667 | HL, nodular sclerosis grade 2 | ||||||||
| 9652 | HL, mixed cellularity, NOS | 909 | 0.41 | (0.38-0.43) | 0.53 | (0.49-0.58) | 0.28 | (0.25-0.32) | |
| 9653 | HL, lymphocyte depletion, NOS | 89 | 0.04 | (0.03-0.05) | 0.05 | (0.04-0.07) | 0.03 | (0.02-0.04) | |
| 9654 | HL, lymphocyte depletion, diffuse fibrosis | ||||||||
| 9655 | HL, lymphocyte depletion, reticular | ||||||||
| Mature B-cell neoplasms | 42 855 | 19.14 | (18.96-19.32) | 21.30 | (21.03-21.57) | 17.07 | (16.83-17.31) | ||
| SBLL/CLL | 9670 | Malignant lymphoma, small B-cell lymphocytic, NOS | 11 019 | 4.92 | (4.83-5.01) | 5.87 | (5.73-6.02) | 4.01 | (3.90-4.13) |
| 9823 | B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma | ||||||||
| Immunoproliferative diseases | 9671 | Malignant lymphoma, lymphoplasmacytic | 1859 | 0.83 | (0.79-0.87) | 1.00 | (0.94-1.06) | 0.67 | (0.63-0.72) |
| 9760 | Immunoproliferative disease, NOS | ||||||||
| 9761 | Waldenström macroglobulinemia | ||||||||
| 9762 | Heavy chain disease, NOS | ||||||||
| Mantle cell/centrocytic lymphoma | 9673 | Mantle cell lymphoma | 1012 | 0.45 | (0.42-0.48) | 0.64 | (0.60-0.69) | 0.27 | (0.24-0.30) |
| Follicular B-cell lymphoma | 9690 | Follicular lymphoma, NOS | 4881 | 2.18 | (2.12-2.24) | 2.10 | (2.01-2.19) | 2.26 | (2.17-2.35) |
| 9691 | Follicular lymphoma, grade 2 | ||||||||
| 9695 | Follicular lymphoma, grade 1 | ||||||||
| 9698 | Follicular lymphoma, grade 3 | ||||||||
| Diffuse B-cell lymphoma | 9675 | Malignant lymphoma, mixed small and large cell, diffuse (obsolete) | 8538 | 3.81 | (3.73-3.89) | 4.06 | (3.95-4.19) | 3.57 | (3.46-3.68) |
| 9678 | Primary effusion lymphoma | ||||||||
| 9679 | Mediastinal large B-cell lymphoma | ||||||||
| 9680 | Malignant lymphoma, large B-cell, diffuse, NOS | ||||||||
| 9684 | Malignant lymphoma, large B-cell, diffuse, immunoblastic, NOS | ||||||||
| Burkitt lymphoma/leukemia | 9687 | Burkitt lymphoma, NOS | 488 | 0.22 | (0.20-0.24) | 0.31 | (0.27-0.34) | 0.13 | (0.11-0.16) |
| 9826 | Burkitt cell leukemia | ||||||||
| Marginal zone lymphoma | 9689 | Splenic marginal zone B-cell lymphoma | 950 | 0.42 | (0.40-0.45) | 0.40 | (0.36-0.44) | 0.45 | (0.41-0.49) |
| 9699 | Marginal zone B-cell lymphoma, NOS/mucosa-associated lymphoid tissue lymphoma | ||||||||
| 9764 | Immunoproliferative small intestinal disease (Mediterranean lymphoma) | ||||||||
| Mature B-cell leukemia | 9833 | Prolymphocytic leukemia, B-cell type | 652 | 0.29 | (0.27-0.31) | 0.46 | (0.42-0.50) | 0.13 | (0.11-0.15) |
| 9940 | Hairy cell leukemia | ||||||||
| Plasma cell neoplasms | 13 456 | 6.01 | (5.91-6.11) | 6.46 | (6.31-6.61) | 5.58 | (5.44-5.72) | ||
| 9732 | Multiple myeloma | 12 192 | 5.44 | (5.35-5.54) | 5.85 | (5.70-5.99) | 5.06 | (4.93-5.19) | |
| 9733 | Plasma cell leukemia | 92 | 0.04 | (0.03-0.05) | 0.04 | (0.03-0.05) | 0.05 | (0.03-0.06) | |
| 9731 | Plasmacytoma, NOS | 1172 | 0.52 | (0.49-0.55) | 0.58 | (0.53-0.62) | 0.47 | (0.43-0.51) | |
| 9734 | Plasmacytoma, extramedullary | ||||||||
| Mature T-cell and NK-cell neoplasms | 2527 | 1.13 | (1.08-1.17) | 1.41 | (1.34-1.48) | 0.86 | (0.81-0.92) | ||
| Cutaneous T-cell lymphoma | 9700 | Mycosis fungoides | 1208 | 0.54 | (0.51-0.57) | 0.68 | (0.64-0.73) | 0.40 | (0.37-0.44) |
| 9701 | Sézary syndrome | ||||||||
| 9708 | Subcutaneous T panniculitis-like T-cell lymphoma | ||||||||
| 9709 | Cutaneous T-cell lymphoma, NOS | ||||||||
| 9718 | Primary cutaneous CD30+ T-cell lymphoproliferative disorder | ||||||||
| Other T-cell lymphomas | 9702 | Mature T-cell lymphoma, NOS | 1319 | 0.59 | (0.56-0.62) | 0.72 | (0.67-0.78) | 0.46 | (0.42-0.50) |
| 9705 | Angioimmunoblastic T-cell lymphoma | ||||||||
| 9714 | Anaplastic large cell lymphoma, T-cell and null cell type | ||||||||
| 9716 | Hepatosplenic γδ cell lymphoma | ||||||||
| 9717 | Intestinal T-cell lymphoma | ||||||||
| 9948 | Aggressive NK-cell leukemia | ||||||||
| 9719 | NK/T-cell lymphoma, nasal and nasal-type | ||||||||
| 9827 | Adult T-cell leukemia/lymphoma (HTLV-1 positive) | ||||||||
| 9831 | T-cell large granular lymphocytic leukemia | ||||||||
| 9834 | Prolymphocytic leukemia, T-cell type | ||||||||
| Lymphoblastic lymphoma/acute (precursor cell) lymphatic leukemia | 2863 | 1.28 | (1.23-1.33) | 1.44 | (1.37-1.51) | 1.12 | (1.06-1.19) | ||
| B-cell | 9728 | Precursor B-cell lymphoblastic lymphoma | 190 | 0.08 | (0.07-0.10) | 0.09 | (0.07-0.11) | 0.08 | (0.06-0.10) |
| 9836 | Precursor B-cell lymphoblastic leukemia | ||||||||
| T-cell | 9729 | Precursor T-cell lymphoblastic lymphoma | 64 | 0.03 | (0.02-0.04) | 0.04 | (0.03-0.06) | 0.01 | (0.01-0.02) |
| 9837 | Precursor T-cell lymphoblastic leukemia | ||||||||
| NOS | 9727 | Precursor cell lymphoblastic lymphoma, NOS | 2609 | 1.17 | (1.12-1.21) | 1.31 | (1.24-1.38) | 1.03 | (0.97-1.09) |
| 9835 | Precursor cell lymphoblastic leukemia, NOS | ||||||||
| Unknown lymphoid neoplasms | 12 547 | 5.60 | (5.51-5.70) | 5.87 | (5.72-6.01) | 5.35 | (5.22-5.49) | ||
| Lymphoma, NOS | 9590 | Malignant lymphoma, NOS | 4803 | 2.14 | (2.08-2.21) | 2.21 | (2.12-2.30) | 2.09 | (2.00-2.17) |
| NHL, NOS | 9591 | Malignant lymphoma, NHL, NOS | 7450 | 3.33 | (3.25-3.40) | 3.51 | (3.40-3.62) | 3.15 | (3.05-3.25) |
| Lymphatic leukemia, NOS | 9820 | Lymphoid leukemia, NOS | 294 | 0.13 | (0.12-0.15) | 0.15 | (0.13-0.17) | 0.12 | (0.10-0.14) |
| 9832 | Prolymphocytic leukemia, NOS | ||||||||
| All lymphoid malignancies* | 66 371 | 29.64 | (29.41-29.86) | 32.83 | (32.49-33.17) | 26.59 | (26.29-26.89) | ||
| HAEMACARE groupings . | ICD-O-3 code . | ICD-O-3 description . | No. of cases . | All . | Males . | Females . | |||
|---|---|---|---|---|---|---|---|---|---|
| IR . | 95% CI . | IR . | 95% CI . | IR . | 95% CI . | ||||
| HL | 5571 | 2.49 | (2.42-2.55) | 2.81 | (2.71-2.91) | 2.18 | (2.09-2.26) | ||
| HL, nodular lymphocyte predominance | 9659 | HL, nodular lymphocyte predominance | 195 | 0.09 | (0.08-0.10) | 0.12 | (0.10-0.15) | 0.05 | (0.04-0.07) |
| Classic HL | 5376 | 2.40 | (2.34-2.47) | 2.69 | (2.59-2.79) | 2.12 | (2.04-2.21) | ||
| 9650 | HL, NOS | 996 | 0.44 | (0.42-0.47) | 0.52 | (0.48-0.57) | 0.37 | (0.34-0.41) | |
| 9661 | Hodgkin granuloma (obsolete) | ||||||||
| 9662 | Hodgkin sarcoma (obsolete) | ||||||||
| 9651 | HL, lymphocyte rich | 217 | 0.10 | (0.08-0.11) | 0.13 | (0.11-0.15) | 0.07 | (0.05-0.08) | |
| 9663 | HL, nodular sclerosis, NOS | 3165 | 1.41 | (1.36-1.46) | 1.45 | (1.38-1.53) | 1.37 | (1.31-1.44) | |
| 9664 | HL, nodular sclerosis cellular phase | ||||||||
| 9665 | HL, nodular sclerosis grade 1 | ||||||||
| 9667 | HL, nodular sclerosis grade 2 | ||||||||
| 9652 | HL, mixed cellularity, NOS | 909 | 0.41 | (0.38-0.43) | 0.53 | (0.49-0.58) | 0.28 | (0.25-0.32) | |
| 9653 | HL, lymphocyte depletion, NOS | 89 | 0.04 | (0.03-0.05) | 0.05 | (0.04-0.07) | 0.03 | (0.02-0.04) | |
| 9654 | HL, lymphocyte depletion, diffuse fibrosis | ||||||||
| 9655 | HL, lymphocyte depletion, reticular | ||||||||
| Mature B-cell neoplasms | 42 855 | 19.14 | (18.96-19.32) | 21.30 | (21.03-21.57) | 17.07 | (16.83-17.31) | ||
| SBLL/CLL | 9670 | Malignant lymphoma, small B-cell lymphocytic, NOS | 11 019 | 4.92 | (4.83-5.01) | 5.87 | (5.73-6.02) | 4.01 | (3.90-4.13) |
| 9823 | B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma | ||||||||
| Immunoproliferative diseases | 9671 | Malignant lymphoma, lymphoplasmacytic | 1859 | 0.83 | (0.79-0.87) | 1.00 | (0.94-1.06) | 0.67 | (0.63-0.72) |
| 9760 | Immunoproliferative disease, NOS | ||||||||
| 9761 | Waldenström macroglobulinemia | ||||||||
| 9762 | Heavy chain disease, NOS | ||||||||
| Mantle cell/centrocytic lymphoma | 9673 | Mantle cell lymphoma | 1012 | 0.45 | (0.42-0.48) | 0.64 | (0.60-0.69) | 0.27 | (0.24-0.30) |
| Follicular B-cell lymphoma | 9690 | Follicular lymphoma, NOS | 4881 | 2.18 | (2.12-2.24) | 2.10 | (2.01-2.19) | 2.26 | (2.17-2.35) |
| 9691 | Follicular lymphoma, grade 2 | ||||||||
| 9695 | Follicular lymphoma, grade 1 | ||||||||
| 9698 | Follicular lymphoma, grade 3 | ||||||||
| Diffuse B-cell lymphoma | 9675 | Malignant lymphoma, mixed small and large cell, diffuse (obsolete) | 8538 | 3.81 | (3.73-3.89) | 4.06 | (3.95-4.19) | 3.57 | (3.46-3.68) |
| 9678 | Primary effusion lymphoma | ||||||||
| 9679 | Mediastinal large B-cell lymphoma | ||||||||
| 9680 | Malignant lymphoma, large B-cell, diffuse, NOS | ||||||||
| 9684 | Malignant lymphoma, large B-cell, diffuse, immunoblastic, NOS | ||||||||
| Burkitt lymphoma/leukemia | 9687 | Burkitt lymphoma, NOS | 488 | 0.22 | (0.20-0.24) | 0.31 | (0.27-0.34) | 0.13 | (0.11-0.16) |
| 9826 | Burkitt cell leukemia | ||||||||
| Marginal zone lymphoma | 9689 | Splenic marginal zone B-cell lymphoma | 950 | 0.42 | (0.40-0.45) | 0.40 | (0.36-0.44) | 0.45 | (0.41-0.49) |
| 9699 | Marginal zone B-cell lymphoma, NOS/mucosa-associated lymphoid tissue lymphoma | ||||||||
| 9764 | Immunoproliferative small intestinal disease (Mediterranean lymphoma) | ||||||||
| Mature B-cell leukemia | 9833 | Prolymphocytic leukemia, B-cell type | 652 | 0.29 | (0.27-0.31) | 0.46 | (0.42-0.50) | 0.13 | (0.11-0.15) |
| 9940 | Hairy cell leukemia | ||||||||
| Plasma cell neoplasms | 13 456 | 6.01 | (5.91-6.11) | 6.46 | (6.31-6.61) | 5.58 | (5.44-5.72) | ||
| 9732 | Multiple myeloma | 12 192 | 5.44 | (5.35-5.54) | 5.85 | (5.70-5.99) | 5.06 | (4.93-5.19) | |
| 9733 | Plasma cell leukemia | 92 | 0.04 | (0.03-0.05) | 0.04 | (0.03-0.05) | 0.05 | (0.03-0.06) | |
| 9731 | Plasmacytoma, NOS | 1172 | 0.52 | (0.49-0.55) | 0.58 | (0.53-0.62) | 0.47 | (0.43-0.51) | |
| 9734 | Plasmacytoma, extramedullary | ||||||||
| Mature T-cell and NK-cell neoplasms | 2527 | 1.13 | (1.08-1.17) | 1.41 | (1.34-1.48) | 0.86 | (0.81-0.92) | ||
| Cutaneous T-cell lymphoma | 9700 | Mycosis fungoides | 1208 | 0.54 | (0.51-0.57) | 0.68 | (0.64-0.73) | 0.40 | (0.37-0.44) |
| 9701 | Sézary syndrome | ||||||||
| 9708 | Subcutaneous T panniculitis-like T-cell lymphoma | ||||||||
| 9709 | Cutaneous T-cell lymphoma, NOS | ||||||||
| 9718 | Primary cutaneous CD30+ T-cell lymphoproliferative disorder | ||||||||
| Other T-cell lymphomas | 9702 | Mature T-cell lymphoma, NOS | 1319 | 0.59 | (0.56-0.62) | 0.72 | (0.67-0.78) | 0.46 | (0.42-0.50) |
| 9705 | Angioimmunoblastic T-cell lymphoma | ||||||||
| 9714 | Anaplastic large cell lymphoma, T-cell and null cell type | ||||||||
| 9716 | Hepatosplenic γδ cell lymphoma | ||||||||
| 9717 | Intestinal T-cell lymphoma | ||||||||
| 9948 | Aggressive NK-cell leukemia | ||||||||
| 9719 | NK/T-cell lymphoma, nasal and nasal-type | ||||||||
| 9827 | Adult T-cell leukemia/lymphoma (HTLV-1 positive) | ||||||||
| 9831 | T-cell large granular lymphocytic leukemia | ||||||||
| 9834 | Prolymphocytic leukemia, T-cell type | ||||||||
| Lymphoblastic lymphoma/acute (precursor cell) lymphatic leukemia | 2863 | 1.28 | (1.23-1.33) | 1.44 | (1.37-1.51) | 1.12 | (1.06-1.19) | ||
| B-cell | 9728 | Precursor B-cell lymphoblastic lymphoma | 190 | 0.08 | (0.07-0.10) | 0.09 | (0.07-0.11) | 0.08 | (0.06-0.10) |
| 9836 | Precursor B-cell lymphoblastic leukemia | ||||||||
| T-cell | 9729 | Precursor T-cell lymphoblastic lymphoma | 64 | 0.03 | (0.02-0.04) | 0.04 | (0.03-0.06) | 0.01 | (0.01-0.02) |
| 9837 | Precursor T-cell lymphoblastic leukemia | ||||||||
| NOS | 9727 | Precursor cell lymphoblastic lymphoma, NOS | 2609 | 1.17 | (1.12-1.21) | 1.31 | (1.24-1.38) | 1.03 | (0.97-1.09) |
| 9835 | Precursor cell lymphoblastic leukemia, NOS | ||||||||
| Unknown lymphoid neoplasms | 12 547 | 5.60 | (5.51-5.70) | 5.87 | (5.72-6.01) | 5.35 | (5.22-5.49) | ||
| Lymphoma, NOS | 9590 | Malignant lymphoma, NOS | 4803 | 2.14 | (2.08-2.21) | 2.21 | (2.12-2.30) | 2.09 | (2.00-2.17) |
| NHL, NOS | 9591 | Malignant lymphoma, NHL, NOS | 7450 | 3.33 | (3.25-3.40) | 3.51 | (3.40-3.62) | 3.15 | (3.05-3.25) |
| Lymphatic leukemia, NOS | 9820 | Lymphoid leukemia, NOS | 294 | 0.13 | (0.12-0.15) | 0.15 | (0.13-0.17) | 0.12 | (0.10-0.14) |
| 9832 | Prolymphocytic leukemia, NOS | ||||||||
| All lymphoid malignancies* | 66 371 | 29.64 | (29.41-29.86) | 32.83 | (32.49-33.17) | 26.59 | (26.29-26.89) | ||
Eight cases of composite HL and NHL (ICD-O-3 code 9596) not shown in Table 2 have been included in the totals.
T-NK-cell neoplasms were divided into cutaneous and other T-cell neoplasms. Unknown lymphoid neoplasms were separated into lymphoma NOS, NHL NOS, and lymphatic leukemia NOS.
Myeloid malignancies (Table 3) were grouped into 5 large categories: acute myeloid leukemia (AML), myeloproliferative neoplasms, myelodysplastic syndrome, myelodysplastic/myeloproliferative neoplasms, and unknown myeloid neoplasms. AML was subdivided into 5 subgroups, in accord with WHO indications. Myeloproliferative neoplasms were subdivided into chronic myeloid leukemia (CML) and other morphologic subgroups (other myeloproliferative neoplasms). Unknown myeloid neoplasms were divided into leukemia NOS and myeloid leukemia NOS.
Number of cases and crude incidence rate (IR) per 100 000 for myeloid malignancies diagnosed in 2000-2002 archived in 44 European CRs by sex and morphologic type
| HAEMACARE groupings . | ICD-O-3 code . | ICD-O-3 description . | No. of cases . | Total . | Males . | Females . | |||
|---|---|---|---|---|---|---|---|---|---|
| IR . | 95% CI . | IR . | 95% CI . | IR . | 95% CI . | ||||
| Acute myeloid leukemia* | 8107 | 3.62 | (3.54-3.70) | 3.90 | (3.78-4.02) | 3.35 | (3.25-3.46) | ||
| Subgroup 1 | 9840 | Acute erythroid leukemia | 7545 | 3.37 | (3.29-3.45) | 3.64 | (3.53-3.76) | 3.11 | (3.01-3.21) |
| 9861 | AML, NOS | ||||||||
| 9867 | Acute myelomonocytic leukemia | ||||||||
| 9870 | Acute basophilic leukemia | ||||||||
| 9872 | AML, minimal differentiation | ||||||||
| 9873 | AML without maturation | ||||||||
| 9874 | AML with maturation | ||||||||
| 9891 | Acute monocytic leukemia | ||||||||
| 9910 | Acute megakaryoblastic leukemia | ||||||||
| 9930 | Myeloid sarcoma | ||||||||
| Subgroup 2 | 9866 | Acute promyelocytic leukemia t(15; 17) (q22; q11-12) | 311 | 0.14 | (0.12-0.16) | 0.13 | (0.11-0.15) | 0.15 | (0.13-0.17) |
| 9871 | AML with abnormal marrow eosinophils | ||||||||
| 9896 | AML, t(8,21) (q22,q22) | ||||||||
| 9897 | AML, 11q23 abnormalities | ||||||||
| Subgroup 3 | 9895 | AML, with multilineage dysplasia | 137 | 0.06 | (0.05-0.07) | 0.07 | (0.06-0.09) | 0.05 | (0.04-0.07) |
| 9984 | Refractory anemia with excess blasts in transformation (obsolete) | ||||||||
| Subgroup 4 | 9931 | Acute panmyelosis with myelofibrosis | 106 | 0.05 | (0.04-0.06) | 0.05 | (0.04-0.07) | 0.04 | (0.03-0.05) |
| Myeloproliferative neoplasms | 7474 | 3.34 | (3.26-3.41) | 3.50 | (3.39-3.62) | 3.18 | (3.08-3.28) | ||
| CML | 9863 | CML, NOS | 2468 | 1.10 | (1.06-1.15) | 1.23 | (1.17-1.30) | 0.98 | (0.92-1.04) |
| 9875 | Chronic myelogenous leukemia, BCR/ABL positive | ||||||||
| Other myeloproliferative neoplasms† | 5006 | 2.24 | (2.17-2.30) | 2.27 | (2.19-2.36) | 2.20 | (2.11-2.29) | ||
| Subgroup 1 | 9950 | Polycythemia vera | 3431 | 1.53 | (1.48-1.58) | 1.57 | (1.49-1.64) | 1.50 | (1.43-1.57) |
| 9961 | Myelosclerosis with myeloid metaplasia | ||||||||
| 9962 | Essential thrombocythemia | ||||||||
| 9963 | Chronic neutrophilic leukemia | ||||||||
| 9964 | Hypereosinophilic syndrome | ||||||||
| Subgroup 2 | 9960 | Chronic myeloproliferative disease, NOS | 1546 | 0.69 | (0.66-0.73) | 0.69 | (0.64-0.74) | 0.69 | (0.64-0.74) |
| Myelodysplastic syndrome | 4074 | 1.82 | (1.76-1.88) | 2.03 | (1.95-2.12) | 1.62 | (1.54-1.69) | ||
| 9980 | Refractory anemia | ||||||||
| 9982 | Refractory anemia with sideroblasts | ||||||||
| 9983 | Refractory anemia with excess blasts | ||||||||
| 9985 | Refractory cytopenia with multilineage dysplasia | ||||||||
| 9986 | Myelodysplastic syndrome 5q deletion | ||||||||
| 9989 | Myelodysplastic syndrome, NOS | ||||||||
| Myelodysplastic/myeloproliferative neoplasms | 776 | 0.35 | (0.32-0.37) | 0.42 | (0.38-0.46) | 0.28 | (0.25-0.31) | ||
| 9945 | Chronic myelomonocytic leukemia | ||||||||
| 9876 | Atypical CML, BCR/ABL-1 negative | ||||||||
| 9946 | Juvenile myelomonocytic leukemia | ||||||||
| 9975 | Myelodysplastic/myeloproliferative neoplasm, unclassifiable | ||||||||
| Unknown myeloid neoplasms | 1365 | 0.61 | (0.58-0.64) | 0.66 | (0.61-0.71) | 0.56 | (0.52-0.61) | ||
| Leukemia, NOS | 9800 | Leukemia, NOS | 1010 | 0.45 | (0.42-0.48) | 0.48 | (0.44-0.53) | 0.42 | (0.38-0.46) |
| 9801 | Acute leukemia, NOS | ||||||||
| 9805 | Acute leukemia, ambiguous lineage | ||||||||
| Myeloid leukemia, NOS | 9860 | Myeloid leukemia, NOS | 355 | 0.16 | (0.14-0.18) | 0.17 | (0.15-0.20) | 0.14 | (0.12-0.17) |
| All myeloid malignancies | 21 796 | 9.73 | (9.60-9.86) | 10.51 | (10.32-10.70) | 8.99 | (8.82-9.17) | ||
| HAEMACARE groupings . | ICD-O-3 code . | ICD-O-3 description . | No. of cases . | Total . | Males . | Females . | |||
|---|---|---|---|---|---|---|---|---|---|
| IR . | 95% CI . | IR . | 95% CI . | IR . | 95% CI . | ||||
| Acute myeloid leukemia* | 8107 | 3.62 | (3.54-3.70) | 3.90 | (3.78-4.02) | 3.35 | (3.25-3.46) | ||
| Subgroup 1 | 9840 | Acute erythroid leukemia | 7545 | 3.37 | (3.29-3.45) | 3.64 | (3.53-3.76) | 3.11 | (3.01-3.21) |
| 9861 | AML, NOS | ||||||||
| 9867 | Acute myelomonocytic leukemia | ||||||||
| 9870 | Acute basophilic leukemia | ||||||||
| 9872 | AML, minimal differentiation | ||||||||
| 9873 | AML without maturation | ||||||||
| 9874 | AML with maturation | ||||||||
| 9891 | Acute monocytic leukemia | ||||||||
| 9910 | Acute megakaryoblastic leukemia | ||||||||
| 9930 | Myeloid sarcoma | ||||||||
| Subgroup 2 | 9866 | Acute promyelocytic leukemia t(15; 17) (q22; q11-12) | 311 | 0.14 | (0.12-0.16) | 0.13 | (0.11-0.15) | 0.15 | (0.13-0.17) |
| 9871 | AML with abnormal marrow eosinophils | ||||||||
| 9896 | AML, t(8,21) (q22,q22) | ||||||||
| 9897 | AML, 11q23 abnormalities | ||||||||
| Subgroup 3 | 9895 | AML, with multilineage dysplasia | 137 | 0.06 | (0.05-0.07) | 0.07 | (0.06-0.09) | 0.05 | (0.04-0.07) |
| 9984 | Refractory anemia with excess blasts in transformation (obsolete) | ||||||||
| Subgroup 4 | 9931 | Acute panmyelosis with myelofibrosis | 106 | 0.05 | (0.04-0.06) | 0.05 | (0.04-0.07) | 0.04 | (0.03-0.05) |
| Myeloproliferative neoplasms | 7474 | 3.34 | (3.26-3.41) | 3.50 | (3.39-3.62) | 3.18 | (3.08-3.28) | ||
| CML | 9863 | CML, NOS | 2468 | 1.10 | (1.06-1.15) | 1.23 | (1.17-1.30) | 0.98 | (0.92-1.04) |
| 9875 | Chronic myelogenous leukemia, BCR/ABL positive | ||||||||
| Other myeloproliferative neoplasms† | 5006 | 2.24 | (2.17-2.30) | 2.27 | (2.19-2.36) | 2.20 | (2.11-2.29) | ||
| Subgroup 1 | 9950 | Polycythemia vera | 3431 | 1.53 | (1.48-1.58) | 1.57 | (1.49-1.64) | 1.50 | (1.43-1.57) |
| 9961 | Myelosclerosis with myeloid metaplasia | ||||||||
| 9962 | Essential thrombocythemia | ||||||||
| 9963 | Chronic neutrophilic leukemia | ||||||||
| 9964 | Hypereosinophilic syndrome | ||||||||
| Subgroup 2 | 9960 | Chronic myeloproliferative disease, NOS | 1546 | 0.69 | (0.66-0.73) | 0.69 | (0.64-0.74) | 0.69 | (0.64-0.74) |
| Myelodysplastic syndrome | 4074 | 1.82 | (1.76-1.88) | 2.03 | (1.95-2.12) | 1.62 | (1.54-1.69) | ||
| 9980 | Refractory anemia | ||||||||
| 9982 | Refractory anemia with sideroblasts | ||||||||
| 9983 | Refractory anemia with excess blasts | ||||||||
| 9985 | Refractory cytopenia with multilineage dysplasia | ||||||||
| 9986 | Myelodysplastic syndrome 5q deletion | ||||||||
| 9989 | Myelodysplastic syndrome, NOS | ||||||||
| Myelodysplastic/myeloproliferative neoplasms | 776 | 0.35 | (0.32-0.37) | 0.42 | (0.38-0.46) | 0.28 | (0.25-0.31) | ||
| 9945 | Chronic myelomonocytic leukemia | ||||||||
| 9876 | Atypical CML, BCR/ABL-1 negative | ||||||||
| 9946 | Juvenile myelomonocytic leukemia | ||||||||
| 9975 | Myelodysplastic/myeloproliferative neoplasm, unclassifiable | ||||||||
| Unknown myeloid neoplasms | 1365 | 0.61 | (0.58-0.64) | 0.66 | (0.61-0.71) | 0.56 | (0.52-0.61) | ||
| Leukemia, NOS | 9800 | Leukemia, NOS | 1010 | 0.45 | (0.42-0.48) | 0.48 | (0.44-0.53) | 0.42 | (0.38-0.46) |
| 9801 | Acute leukemia, NOS | ||||||||
| 9805 | Acute leukemia, ambiguous lineage | ||||||||
| Myeloid leukemia, NOS | 9860 | Myeloid leukemia, NOS | 355 | 0.16 | (0.14-0.18) | 0.17 | (0.15-0.20) | 0.14 | (0.12-0.17) |
| All myeloid malignancies | 21 796 | 9.73 | (9.60-9.86) | 10.51 | (10.32-10.70) | 8.99 | (8.82-9.17) | ||
Eight cases of therapy-related AML, NOS, and therapy-related myelodysplastic syndrome, NOS (ICD-O-3 codes 9920 and 9987, respectively) not shown in Table 3 were included with acute myeloid leukemia.
Twenty-nine cases of mastocytoma NOS/mast cell sarcoma, malignant mastocytosis, and mast cell leukemia (ICD-O-3 codes 9740, 9741, and 9742, respectively) not shown in Table 3 were included with other myeloproliferative neoplasms.
Statistical analysis
We estimated crude incidence rates per 100 000 with 95% confidence intervals (95% confidence interval [CI]) for each sex and each morphologic subcategory (as shown in the left column of Tables 2, 3) by CR, using CR area-specific populations.10 We also estimated incidence according to age at diagnosis, grouped into 6 categories: 0-14, 15-44, 45-54, 55-64, 65-74, and 75-99 years. Finally, we estimated, using the direct method, age-standardized incidence rates per 100 000 for each CR area, and for the entire dataset, for each of the HAEMACARE disease categories (5 lymphoid and 5 myeloid) defined in “HM categories,” considering the European population as standard. The calculations and analyses were carried out using the SEER STAT software package, Version 6.4.4. (Information Management Services Inc, and Surveillance Research Program of the Division of Cancer Control and Population Sciences, National Cancer Institute).
Results
Table 1 shows some indicators of data quality as well as mean and median ages at diagnosis. Overall, 92.7% (range, 64.3%-100.0%) of cases were microscopically verified, with less than 80.0% microscopically verified in 6 CRs representing 9% of the average population covered. Overall, 3.2% (range, 0.0%-17.3%) of cases were known by death certificate only, or discovered at autopsy, with more than 5% in 6 CRs, representing 27% of the average population covered. Overall, 17.6% (range, 0.8%-50.7%) of cases were NOS: 15.9% of lymphoid cases (lymphoma NOS, NHL NOS, and lymphatic leukemia NOS) and 1.6% of myeloid cases (leukemia NOS; acute leukemia NOS; acute leukemia, ambiguous lineage; and myeloid leukemia NOS). Mean overall age was 64 years, range of means 60 years (Eastern Europe) to 65 years (Northern Europe and United Kingdom and Ireland); median age was 69 years, range of medians 65 (Eastern Europe) to 70 years (Northern Europe). A total of 66 371 lymphoid malignancies incident in 2000-2002 in the 44 CRs were included in the analyses (Table 2). The overall crude incidence rate of lymphoid malignancies was 29.64 per 100 000: 32.83 for males and 26.59 for females.
Considering specific lymphoid malignancy subgroups, the overall crude incidence rate of HL was 2.49, the commonest subtype being classic HL with nodular sclerosis. The overall crude incidence rate of mature B-cell neoplasms was 19.14. The most common subtypes were SBLL/CLL (4.92), diffuse B-cell lymphoma (3.81), and follicular B-cell lymphoma (2.18). Immunoproliferative diseases, mantle cell/centrocytic lymphoma, Burkitt lymphoma/leukemia, marginal zone lymphoma, and mature B-cell leukemias (prolymphocytic and hairy cell) all had crude incidence rates of less than 1, whereas the incidence of plasma cell neoplasms, mainly multiple myeloma, was fairly high at 6.01.
The overall incidence of T-NK-cell neoplasms was 1.13, approximately one-half of which were cutaneous T-cell lymphomas and the other half T-cell lymphomas. The crude incidence of LL/ALL was 1.28, for most of which (1.17) the B versus T type was unknown (ie, NOS). The crude incidence of unknown types of lymphoid neoplasm was 5.60, including NHL NOS at 3.33, based on 7450 cases; and lymphoma NOS at 2.14, based on 4803 cases. For most lymphoid malignancies, crude incidence was higher in men than in women.
Table 3 shows crude incidence rates for myeloid malignancies. A total of 21 796 myeloid malignancies, diagnosed in 2000-2002, were archived in the 44 European CRs. The overall crude incidence rate was 9.73: 10.51 in men and 8.99 in women.
Considering specific myeloid subgroups, the overall incidence rate of AML was 3.62. The most common AML was subgroup 1 (Table 3), with incidence 3.37, which includes AML NOS, and malignancies arising from various other myeloid lineages, such as myelomonocytic, monocytic, basophilic, erythroid, and megakaryoblastic forms. The incidence of subgroup 2, composed of promyelocytic leukemia and other AMLs with recurrent genetic abnormalities, was 0.14. There were 137 cases in subgroup 3, including AML with multilineage dysplasia and refractory anemia with excess blasts in transformation; 106 cases in subgroup 4, including acute panmyelosis with myelofibrosis and only 8 cases of therapy-related AML, NOS, or therapy-related myelodysplastic syndrome, NOS (not shown in Table 3).
There were 7474 incident cases of myeloproliferative neoplasms, with overall incidence 3.34. This category included CML (crude incidence 1.10) and other myeloproliferative neoplasms (2.24). The crude incidence of myelodysplastic syndrome was 1.82, whereas for myelodysplastic/myeloproliferative neoplasms mainly represented by chronic myelomonocytic leukemia (756 of 776 cases), incidence was 0.35.
The incidence of leukemia NOS was 0.45, based on 1010 cases and incidence of myeloid leukemia NOS was 0.16, based on 355 cases.
Like lymphoid malignancies, for most myeloid malignancies incidence was higher in males than females.
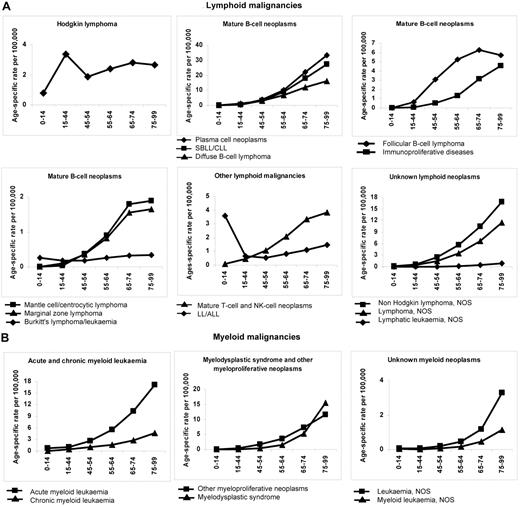
Figure 1 shows age-specific incidence rates (per 100 000) for lymphoid and myeloid malignancies, respectively, by broad HAEMACARE groupings and by age class. Incidence generally increased with age, reaching a maximum at 75-99 years. Notable exceptions were HL and LL/ALL: For HL, incidence was bimodal, peaking at 15-44 years (3.35; 95% CI, 3.23-3.47) and 65-74 years (2.80; 95% CI, 2.56-3.05). For LL/ALL, incidence was high at 0-14 years (3.59; 95% CI, 3.40-4.78), decreased to 0.53 (95% CI, 0.45-0.61) at 45-54 years and increased with advancing age thereafter (to 1.45; 95% CI, 1.27-1.65, at 75-99 years). The incidence trend with age for Burkitt lymphoma/leukemia also showed a trough, with a peak in childhood (0.26, 95% CI, 0.22-0.32), which declined at 15-44 years and 45-54 years (0.17; 95% CI, 0.14-0.19 and 0.17; 95% CI, 0.13-0.23) and increased subsequently, to 0.33 (95% CI, 0.25-0.43) at 75-99 years.
Age-specific incidence rates (per 100 000) for HMs diagnosed in 2000-2002 and archived by 44 European CRs by age class and morphologic type (HAEMACARE groupings). (A) Lymphoid malignancies. (B) Myeloid malignancies.
Age-specific incidence rates (per 100 000) for HMs diagnosed in 2000-2002 and archived by 44 European CRs by age class and morphologic type (HAEMACARE groupings). (A) Lymphoid malignancies. (B) Myeloid malignancies.
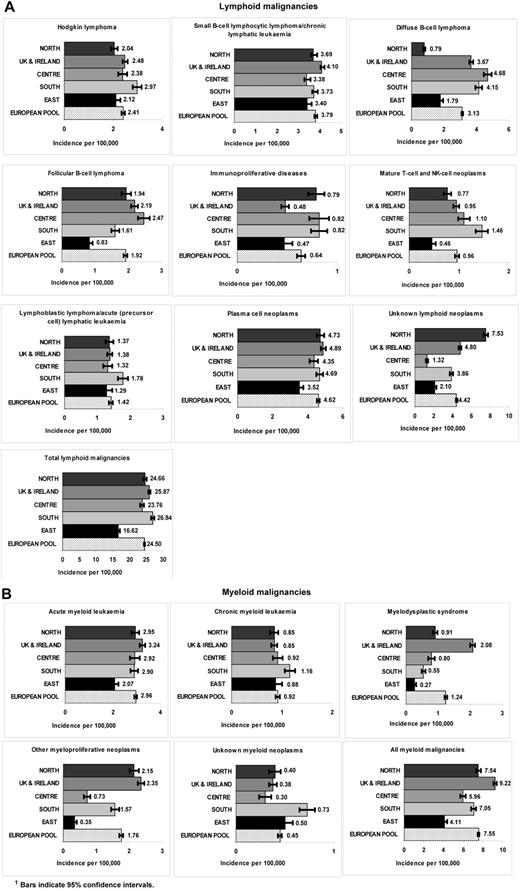
Figure 2 shows age-standardized incidence rates by European region for broad HAEMACARE groupings. Considering first lymphoid malignancies, with reference to the European average, HL incidence was significantly higher in Southern Europe (2.97) and significantly lower in Eastern Europe (2.12) and Northern Europe (2.04). For SBLL/CLL, incidence rates were closely similar across the 5 regions. For diffuse B-cell lymphoma, incidence was significantly lower in Eastern Europe (1.79) and Northern Europe (0.79), with no remarkable differences between other European regions. For follicular B-cell lymphoma, incidence was significantly lower in Eastern Europe (0.83) and significantly higher in Central Europe (2.47) and United Kingdom and Ireland (2.19). For immunoproliferative diseases, incidence was significantly lower than the European average in the United Kingdom and Ireland (0.48) and Eastern Europe (0.47). For T-NK-cell neoplasms, incidence was significantly higher in Southern Europe (1.46) and lower in Eastern Europe (0.46) and Northern Europe (0.77). For LL/ALL, incidence was also significantly higher in Southern Europe (1.78). For plasma cell neoplasms, incidence was significantly lower in Eastern Europe (3.52) and higher in United Kingdom and Ireland (4.89). For lymphoid malignancies of unknown type, incidence was significantly higher in Northern Europe (7.53), whereas for all lymphoid malignancies together, incidence was significantly lower in Eastern Europe (16.62) and higher in Southern Europe (26.84) and United Kingdom and Ireland (25.87).
Age-standardized incidence rates (per 100 000) for HMs diagnosed in 2000-2002 and archived by 44 European CRs by European region and morphologic type (HAEMACARE groupings). (A) Lymphoid malignancies. (B) Myeloid malignancies.
Age-standardized incidence rates (per 100 000) for HMs diagnosed in 2000-2002 and archived by 44 European CRs by European region and morphologic type (HAEMACARE groupings). (A) Lymphoid malignancies. (B) Myeloid malignancies.
Considering now myeloid malignancies, the incidence of AML was significantly lower than the European average in Eastern Europe (2.07) and higher in the United Kingdom and Ireland (3.24). The incidence of CML was significantly higher in Southern Europe (1.16), with no remarkable differences across the other areas. For myelodysplastic syndrome and other myeloproliferative neoplasms, incidence was significantly higher than the European average in United Kingdom and Ireland (2.08 and 2.35, respectively) and lower in Eastern Europe (0.27 and 0.35, respectively). For unknown myeloid neoplasms, incidence was highest in Southern Europe (0.73). For all myeloid malignancies (total), United Kingdom and Ireland had the highest incidence (9.22) and Eastern Europe the lowest (4.11).
Discussion
Incidence is one of the major measures of disease burden in a population (together with prevalence, mortality, and survival) and serves as an important guide the allocation of public health resources. Most previous studies on HM incidence divided the daunting number of HM subtypes into broad categories taking no account of the great variation in prognosis between diseases of similar cell lineage or maturation stage. For epidemiologic and public health purposes, it makes more sense to group diseases (defined by ICD-O-3 code) into categories useful for investigating prognosis and testing etiologic hypotheses because diseases arising from the same cell lineage may have similar etiologies, and are more compatible with clinical classifications than the broad categories used by CRs.
We considered only cases incident in 2000-2002, when the ICD-O-3 classification was being used by all CRs participating in this study. Nevertheless, the availability and quality of morphology data varied between CRs and countries. We therefore further restricted our analysis to CRs that had less than 30% of NOS cases, an arbitrary percentage nonetheless indicating a reasonably satisfactory level of detail of information on morphology. Even with this restriction, however, the numbers of cases with poorly defined morphology (particularly lymphoma NOS and NHL NOS) were relatively high. Centralized revision of slides would have improved the quality of our data, but the resources were not available for such a task.
On the positive side, the high concordance of incidence data with that published in CI51 supports the completeness of our incidence estimates for the HM categories compared.
In agreement with other studies,9,11,12 we found that incidence varied with HM type. Thus, lymphoid malignancies were more common than myeloid malignancies. In addition, for both these disease groupings, incidence increased steadily with advancing age. As for solid cancers, accumulating DNA damage and diminished immune surveillance with age have been suggested as causes of increasing cancer incidence with age.13
In contrast to the general age trend, LL/ALL incidence peaked in children 0-14 years of age, HL incidence peaked in the 15- to 44-year age range, and there was a trough of the incidence of Burkitt lymphoma/leukemia at 15-44 years. The childhood peak in LL/ALL is well known1,2 and has been related to host susceptibility factors and response to antigens in early or prenatal life,14 to exposure to electromagnetic fields,15 or to exposure to benzene and other hydrocarbons from traffic during intrauterine life and childhood.16 The hypothesis that children are more susceptible than adults to the carcinogenic effects of benzene deserves further investigation.16 Paternal smoking has been significantly linked to childhood LL/ALL, Burkitt lymphoma/leukemia, and AML.17
The bimodal age trend for HL incidence has been noted previously.1,2 It has been suggested that the HL incidence peak in children, which tends to affect children of poorer families, is due to an infectious agent. The peak in young adults, on the other hand, could result from infection by an agent that commonly attacks children in whom it rarely causes HL but is more likely to do so if it affects adolescents or young adults.18 The main candidate proposed as cause of HL (and other HMs) is Epstein-Barr virus.19
We found that HM incidence was generally lower in women than men; this is a well-known phenomenon1,2 and could be in part the result of lower exposure to environmental and occupational risk factors in women than men. Thus, increased risk of lymphoid malignancies has been documented in farmers exposed to pesticides,20,21 in workers in industries using formaldehyde,22 and in those exposed to dioxins.23 Most workers in these sectors are male. In the years before the study period, the greater prevalence of HIV infection in men than women was probably responsible for the higher incidence of NHL in men24,25 ; however, the introduction of aggressive antiretroviral therapies in the mid 1990s appears to have lowered the incidence of NHL in HIV-infected persons.25,26 The higher prevalence of smoking27 and greater alcohol intake in men than women may also contribute to the higher incidence of all HMs in men than in women. However, results of studies on smoking status and NHL risk are conflicting,25 as are results of studies investigating the association between alcohol consumption and myeloid leukemia.28 Some studies indicate that alcohol consumption is associated with reduced risk of NHL.25 The incidence of most cancers (not only HMs) is lower in women than men.29 Cook et al suggested that “universal mechanisms” might increase male susceptibility to cancer.29 They also cited various possible explanatory hypotheses, including those noted earlier in this paragraph and hormonal and genetic differences between men and women. We found that the incidence of most HMs varied considerably across Europe, with lowest rates of both lymphoid and myeloid malignancies in Eastern Europe (Figure 2). As regards lymphoid malignancies, the highest incidence of HL, LL/ALL, and mature T-NK neoplasms was in Southern Europe, and of diffuse and follicular B-cell neoplasms in Central Europe. Conversely, the United Kingdom and Ireland had the highest incidence of AML, myelodysplastic syndrome, and other myeloproliferative neoplasms. High incidence of most lymphoid malignancies in Southern Europe has been reported by other studies.1,2 However, we are not aware of studies that have attempted to correlate known risk factors for HMs with regional variations in incidence (as opposed to incidence hotspots). It is noteworthy that the regional variation in incidence of all lymphoid malignancies was less marked than the variation for specific lymphoid subgroups. This suggests that the geographic variation for lymphoid subgroups may be the result of more coding and diagnostic practices, including variation among pathologists in applying the classification criteria, than regional differences in prevalence of risk factors (and hence real differences in incidence). In Northern Europe, the high incidence of NOS in contrast with the low incidence of diffuse B-cell lymphoma suggests that a substantial fraction of the latter was registered as unknown lymphoid neoplasms.
It is also noteworthy that there was considerably less geographic variation in the incidence of AML and CML than for myelodysplastic syndrome and for other myeloproliferative neoplasms (Figure 2B). For the first 2 entities, diagnostic and classification criteria have been stable for some time, whereas for the latter 2, important changes in classification have occurred.
The high incidence of NOS cases in the elderly suggests lower diagnostic intensity, in turn suggesting inadequate diagnostic workup/care, difficulties in accessing hospitals, or poverty in elderly patients. Elderly patients may also be considered by physicians to have poor prognoses (perhaps because of the frequent presence of comorbidities), and thus receive a suboptimal diagnostic workup.
Our finding of conspicuously low incidence rates for both lymphoid and myeloid malignancies in Eastern Europe is in line with Globocan data.2 This could reflect genuinely low HM incidence in this part of Europe but could also in part be the result of underreporting. When we analyzed age-specific incidence rates (data not shown), we found that for those aged up to 54 years the incidence of all lymphoid malignancies (and also of their main subtypes follicular and diffuse B-cell lymphomas) in Eastern Europe was similar to that in the other parts of Europe, whereas low incidence was conspicuous in the 75- to 99-year age group. Lower incidence in Eastern European elderly patients was particularly marked for LL/ALL, multiple myeloma, myeloproliferative neoplasms, and myelodysplastic syndrome. LL/ALL patients may escape registration because of death; the other 3 conditions can be diagnosed and treated on an outpatient basis and for this reason also probably escape cancer registration. It is possible, therefore, that these diseases are underdiagnosed in the elderly because of less thorough diagnostic investigation.30
Epidemiologic studies using similar HM groupings and including the same ICD-O-3 codes as those used in the present study have been carried out in the United States.9,11,12 The age-standardized incidence rate (per 100 000) for all lymphoid malignancies recorded by 17 SEER CRs in 2001 to 2003 was considerably higher than the age-standardized incidence recorded in our study (33.42 vs 24.50), with greatest differences for diffuse B-cell lymphoma (6.80 vs 3.13) and SBLL/CLL (5.10 vs 3.79), with less marked differences for less common subtypes, which nevertheless were consistently lower in Europe.9
Conversely, the age-standardized incidence rate for all myeloid malignancies in 1992-2001 reported by SEER (12 CRs) was somewhat closer to the age-standardized incidence recorded in our study (6.63 vs 7.55) with lower European figures for AML (3.93 vs 2.96) and CML (1.72 vs 0.92). In 2001-2003, the incidence of myelodysplastic syndrome was considerably higher in SEER than we found in Europe (3.48 vs 1.24).12
The lower incidence of myelodysplastic syndrome in Europe is probably in part the result of European underreporting. The disease mainly affects elderly patients who are less probable to undergo a thorough diagnostic assessment than younger patients.30 Another possible explanation is “excessive” diagnostic activity in the United States, which could inflate incidence, especially in elderly patients in whom these diseases are relatively common.
In addition, myelodysplastic syndrome and the category “other myeloproliferative neoplasms” used to be considered nonmalignant and were not recorded by most European CRs until the adoption of ICD-O-3. Perhaps not all CRs systematically registered these diseases in 2000-2002. Analysis of 13 European CRs with stable incidence rates for myelodysplastic syndrome and other myeloproliferative neoplasms over the study period supports the hypothesis of underreporting in the other CRs. In these 13 CRs, the age-standardized incidence rate was higher than in all 44 CRs for myelodysplastic syndrome (1.97; 95% CI, 1.90-2.04 vs 1.24; 95% CI, 1.20-1.28) and other myeloproliferative neoplasms (2.70; 95% CI, 2.62-2.79 vs 1.76; 95% CI, 1.71-1.81) and closer to the SEER figures. This finding reinforces the idea that the geographic differences in incidence of these diseases in Europe are in part attributable to differences in diagnostic and registration criteria.
In conclusion, our data show that HM incidence by morphologic groupings varies across Europe. Differences in diagnostic and registration criteria across Europe contribute to these differences complicating interpretation, and emphasizing that the quality of HM data needs to be improved. If the quality of data registration improved and the HM classification system remained relatively stable (being flexible enough to accommodate advances in disease understanding without major changes), differences in incidence would increasingly reflect true variations in incidence. However, separating true incidence differences from differences resulting from variations in data quality or diagnostic criteria will always require attentive analysis of the data in relation to knowledge of local conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Don Ward for help with the English and Chiara Margutti and Samba Sowe for editorial support.
This work was supported by D.G. Sanco Public Health & Consumer Protection (grant agreement no. 2004131) and Compagnia di San Paolo di Torino.
Authorship
Contribution: M.S. was the HAEMACARE project leader and contributed to study design, manuscript writing, and study coordination; C.A. and C.T. carried out the statistical analyses; R.D.A and R.C. gave advice on statistical analyses; C.A., C.T., O.V., R.M.-G., M.M., A.S., J.-M.L., and F.B. interpreted results and contributed to writing the manuscript; and the HAEMACARE Working Group provided the population-based incidence data for hematologic malignancies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Milena Sant, Department of Preventive and Predictive Medicine, Unit of Analytical Epidemiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Via Venezian 1, I-20133, Milan, Italy; e-mail: milena.sant@istitutotumori.mi.it.
Appendix: HAEMACARE Working Group
Austria: M. Hackl (National Cancer Registry of Austria); Czech Republic: J. Holub (West Bohemia Cancer Registry); France: M. Maynadie (Côte d'Or Haematological Malignancies Cancer Registry); Germany: B. Holleczek (Saarland Cancer Registry); Iceland: L. Tryggvadottir (National Cancer Registry of Iceland); Ireland: H. Comber (National Cancer Registry of Ireland); Italy: F. Bellù (Alto Adige Cancer Registry), A. Giacomin (Biella Cancer Registry), S. Ferretti (Ferrara Cancer Registry), E. Crocetti (Firenze Cancer Registry), D. Serraino (Friuli Cancer Registry), M. Vercelli (Descriptive Epidemiology Unit, Department of Health Sciences, University of Genoa), M. Federico (Modena Cancer Registry), M. Fusco (Napoli Cancer Registry), M. Michiara (Parma Cancer Registry), R. Tumino (Ragusa Cancer Registry), L. Mangone (Reggio Emilia Cancer Registry), F. Falcini (Romagna Cancer Registry), A. Iannelli (Salerno Cancer Registry), M. Budroni (Sassari Cancer Registry), R. Zanetti (Torino Cancer Registry), S. Piffer (Trento Cancer Registry), F. La Rosa (Umbria Cancer Registry), P. Zambon (Venetian Cancer Registry), M. Sant (Project Leader), C. Allemani, F. Berrino, S. Sowe, C. Tereanu (Fondazione IRCCS Istituto Nazionale dei Tumori, Milan), R. Capocaccia, R. De Angelis, A. Simonetti (National Centre for Epidemiology, Surveillance and Health Promotion, Istituto Superiore di Sanità, Rome); Malta: K. England (National Cancer Registry of Malta); Norway: F. Langmark (National Cancer Registry of Norway); Poland: J. Rachtan (Cracow Cancer Registry), R. Mezyk (Kielce Cancer Registry), M. Zwierko (Warsaw Cancer Registry); Slovakia: M. Ondrusova (National Cancer Registry of the Slovak Republic); Slovenia: M. Primic-Žakelj (National Cancer Registry of Slovenia); Spain: R. Marcos-Gragera (Girona Cancer Registry); Sweden: S. Khan (National Cancer Registry of Sweden); Switzerland: G. Jundt (Basel Cancer Registry), M. Usel (Geneva Cancer Registry), S. M. Ess (St Gall Cancer Registry), A. Bordoni (Ticino Cancer Registry); The Netherlands: O. Visser (Amsterdam Cancer Registry), R. Otter (CCC-Groningen, North Netherlands), J. W. Coebergh (Eindhoven Cancer Registry), S. Siesling (CCC-Stedendriehoek Twente); UK–England: D. Greenberg (Eastern Cancer Registration and Information Centre), N. Easey (Northern and Yorkshire Cancer Registry), M. Roche (Oxford Cancer Intelligence Unit), G. Lawrence (West-Midlands Cancer Intelligence Unit); UK–Northern Ireland: A. Gavin (Northern Ireland Cancer Registry); UK–Scotland: D. H. Brewster (Scottish Cancer Registry); UK–Wales: J. Steward (Welsh Cancer Intelligence & Surveillance Unit).