Abstract
Osteoclast (OC)–mediated lytic bone disease remains a cause of major morbidity in multiple myeloma. Here we demonstrate the critical role of interleukin-17–producing marrow infiltrating lymphocytes (MILs) in OC activation and development of bone lesions in myeloma patients. Unlike MILs from normal bone marrow, myeloma MILs possess few regulatory T cells (Tregs) and demonstrate an interleukin-17 phenotype that enhances OC activation. In univariate analyses of factors mediating bone destruction, levels of cytokines that selectively induce and maintain the Th17 phenotype tightly correlated with the extent of bone disease in myeloma. In contrast, MILs activated under conditions that skew toward a Th1 phenotype significantly reduced formation of mature OC. These findings demonstrate that interleukin-17 T cells are critical to the genesis of myeloma bone disease and that immunologic manipulations shifting MILs from a Th17 to a Th1 phenotype may profoundly diminish lytic bone lesions in multiple myeloma.
Introduction
Multiple myeloma results from the clonal outgrowth of malignant plasma cells and is primarily confined to the bone marrow (BM). In addition to the direct effect of plasma cells on normal hematopoietic function, myeloma-induced lytic bone disease affects the majority of patients and represents a major comorbidity.1-3 The interaction of malignant plasma cells with the surrounding stromal microenvironment mediated by increased receptor activator of nuclear factor-kappa B ligand (RANKL) production and reduced osteoprotegrin secretion plays a critical role in accelerating osteoclast (OC)–mediated bone resorption and destruction.4,5 However, the putative role of additional factors of the bone destructive process is currently unknown.
Immunologically, the BM possesses several unique properties. It is a reservoir of memory T cells with heightened antigen specificity6,7 and is capable of effectively priming naive T-cell responses.8 The non–tumor-bearing marrow also serves as a reservoir of regulatory T cells (Tregs).9 In multiple myeloma, we have previously shown that marrow infiltrating lymphocytes (MILs) are more effectively activated and expanded, possess greater antitumor activity than peripheral blood lymphocytes (PBLs) from the same patients, and thus represent more suitable T cells for adoptive immunotherapy in this disease.10,11 Considering these unique features of MILs, we hypothesized that factors must be present within the tumor microenvironment and absent in the non–tumor-bearing host that are critical in facilitating T-cell activation.
Beyond the classic Th1 and Th2 subsets of helper T cells, Th17 T cells have recently been identified as important in the response to pulmonary and colonic bacterial infections as well as key mediators of pathology in numerous autoimmune conditions, including rheumatoid arthritis, systemic lupus erythematosis, and inflammatory bowel diseases.12 These cells represent a newly defined lineage of T lymphocytes capable of producing interleukin-17 (IL-17) but not interferon-γ (IFN-γ) or IL-4. As such, they are distinct from the canonical Th1 or Th2 lineages.13 Th17 differentiation is mediated by transforming growth factor-β (TGF-β) and IL-6.14 In contrast, increased levels of IL-6 suppress Treg formation.15 The critical role of IL-6 in the suppression of Treg differentiation and promotion of Th17 differentiation15 raises the possibility that myeloma, known to produce significant amounts of IL-6, could alter the Th17/Treg balance. Considering the role of Th17 cells in autoimmune diseases and their recent implication in the bone destruction observed in rheumatoid arthritis,16 we sought to determine whether MILs from myeloma patients produced IL-17 and whether this T-cell population contributed to the osteolytic bone disease in multiple myeloma.
Methods
Samples
BM and PBL samples were obtained from both myeloma patients (N = 68) and normal donors (N = 6) under informed consent in accordance with the Declaration of Helsinki with approval from the Institutional Review Board at Johns Hopkins University. Plasma was removed by centrifugation and stored at −80°C. Lymphocytes were obtained by diluting samples with 1 times Hanks balanced salt solution and recovering the lymphocyte layer from a Ficoll density gradient (Ficoll-Hypque; GE Healthcare). Density gradients were placed in a centrifuge at 309.5g for 30 minutes at room temperature with the brake turned off. All cells were collected to the red blood cell layer washed and counted. Cells were viably frozen and kept in liquid nitrogen until needed for experiments.
Flow cytometry
CD3, CD4, and CD25 antibodies were used for extracellular staining (BD Biosciences). IFN-γ and IL-17 antibodies were used for intracellular staining (eBioscience). For intracellular staining, Leukocyte Activation Cocktail (BD Biosciences) was added at 2 μL/mL of cell culture for 4 hours. Extracellular staining for 10 minutes at 4°C was followed by washing 1 time, permeabilization for 25 minutes (BD Cytofix/Cytoperm Kit), washing 1 time in Permwash, and addition of intracellular antibodies followed for 30 minutes at 16°C followed by a final wash step in fluorescence-activated cell sorter buffer, which consisted of Hanks balanced salt solution, 1% fetal bovine serum, 1% ethylenediaminetetraacetic acid, 1% Na Azide. Cells were analyzed on a FACSCalibur (BD Biosciences).
ELISA
BM and PBL plasma were tested, according to the manufacturer's recommendations, for cytokine concentrations of TGF-B, IL-6, IL-1B, IL-23, IL-17, and macrophage inflammatory protein-1α (MIP-1α) using ELISA kits. (R&D Systems) Briefly, controls were prepared according to the manufacturer's protocols and then plasma was diluted 1:2 or plated neat in triplicates. The assays were carried out as per the manufacturer's instructions.
aMIL culture
BM cells were viably thawed. Surface staining for CD3 was performed to determine the percentage and total number of CD3 cells in the sample. Anti-CD3/anti-CD28 beads (Invitrogen) were added to the sample at a 3 bead: 1 CD3 cell ratio. Cells were cultured as previously described.10 Briefly, MILs were cultured in 96-well plates in X-VIVO 15 medium without the addition of exogenous serum for 5 days. The magnetic beads were subsequently removed by placing the content over a magnet. The cells were then pelleted and harvested.
IL-17 skewing
Peripheral blood or marrow infiltrating T cells were skewed to a IL-17 phenotype as previously described.17 Briefly, viably thawed T cells were coincubated with anti-CD3/anti-CD28 beads at a 3 beads:1 T-cell ratio in 96-well plates. AIM-V (Invitrogen) was supplemented with 10 ng/mL IL-6, 5 ng/mL TGF-β, 10 ng/mL anti–IFN-γ, and 10 ng/mL anti–IL-4 (R&D Systems). Cells were incubated for 3 days at which time they were harvested. Intracellular staining for IL-17 was performed without the addition of phorbol myristate acetate (PMA) and ionomycin.
Osteoclast cultures
Normal donor or myeloma BM was cultured in Dulbecco modified Eagle medium plus 20% horse serum (HS; Invitrogen) in medium alone or with 10 μg/mL macrophage colony-stimulating factor plus 20 μg/mL RANKL (referred to as MR), MR plus 10 μg/mL IFN-γ, MR plus 10 μg/mL IL-17, MR plus activated MILs (aMILs), MR plus IL-17 MILs, or medium alone plus 2 μg/mL anti–IL-17 for 21 days in 96-well, flat-bottom plates, 6 wells were plated for each condition. Fresh medium was exchanged every third day (with or without cytokine supplements according to each well). On day 21, medium was removed and OCs were stained for 23C6 as previously described.18 Briefly, media was aspirated and the cells were fixed with 37% formaldehyde (Sigma-Aldrich) for 20 minutes at room temperature followed by methanol (Sigma-Aldrich). All wells were then washed with PBS plus 1% horse serum (HS). 23C6 antibody (Abcam) was added for 30 minutes at room temperature. Cells were again washed 3 times with PBS plus 1% HS. Biotinylated anti–mouse IgG (Vector Laboratories) was added for 30 minutes at room temperature and wells were again washed with PBS plus 1% HS. ABC Complex (Vector Laboratories) was added for 30 minutes at room temperature, and then wells were then washed with PBS plus 1% HS. A total of 0.1M Tris-HCl buffer (pH 9.5) was added for 10 to 15 minutes until development was detected. OCs were scored as 23C6 positive cells possessing 3 or more nuclei. Data were compiled by averaging the total number of OCs in the 6 wells of each group.
Determination of lytic bone disease in multiple myeloma patients
Skeletal radiologic surveys were examined and evaluated for the presence of lytic lesions. The scoring was modified from that previously described by Mylin et al.19 Briefly, a score of 0 was assigned to patients with no evidence of lytic bone disease, 1 to patients with one lesion less than 1 cm in diameter, 2 to patients with one lesion greater than 1 cm in diameter, 3 to patients with multiple lesions each less than 1 cm in diameter, and 4 to patients with multiple lesions greater than 1 cm in diameter.
Patients and clinical variables
Measurements were available from a total of 40 patients, 25 of which were newly diagnosed. Patients were subdivided into 4 ordered groups (0, 2, 3, and 4) based on the number of osteolytic lesions when the patient entered the study. Groups were outlined as follows: group 0, no detectable lesions as seen on plain radiographs; group 2, one lesion greater than 1 cm of diameter (the one patient with a lesion < 1 cm was aggregated to this group); group 3, multiple lesions of less than 1 cm in diameter; and group 4, multiple lesions greater than 1 cm of diameter. Cytokines levels were measured as described in “ELISA.” Statistical analyses on continuous variables (ie, cytokine levels, calcium levels) were performed using measurements in the original scale and after logarithmic transformation to stabilize measurements variance with similar results.
Statistical methods
The χ2 test of independence was used to evaluate the relationship between each clinical variable of a categorical nature and the clinical classification based on the number of osteolytic lesions. Briefly, contingency tables were constructed and used to compare the classification of bone lesions with various clinical variables (eg, immunoglobulin subtype, treatment, sex, chromosomal abnormalities).
We used the analysis of variance to assess significant differences between the 4 groups of patients as defined by the number of osteolytic lesions for all the continuous variables considered in the study. The analysis of variance models were fitted using measurements in the original scale and after logarithmic transformation with similar results. The model also used each group of patients as the reference group to compute the P values. All analyses were performed using functions and methods implemented in the R statistical environment.20,21
Results
MILs in myeloma possess few regulatory T cells
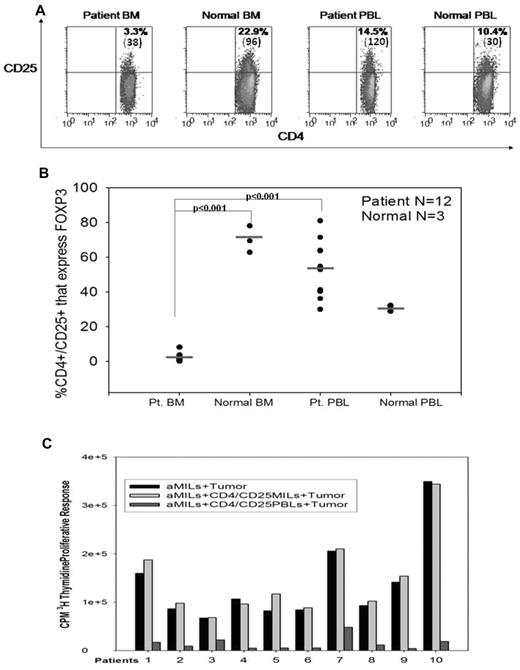
We have previously demonstrated better expansion of MILs from myeloma patients compared with PBLs from the same patients.10 In an effort to understand the mechanisms mediating augmented T-cell responsiveness, we asked whether reduced numbers of Tregs in the BM of myeloma patients could account for the enhanced T-cell proliferation. Paired samples of marrow infiltrating or PBLs from myeloma patients as well as normal donors were stained for CD4 and CD25 as well as the intracellular marker FOXP3. Reminiscent of prior studies demonstrating that normal BM is a reservoir of Tregs,22 we also found that the BM of normal donors showed a higher percentage of CD4/CD25 cells compared with their peripheral blood (PBL). In contrast, in myeloma patients, the percentage of CD4/CD25 cells was higher in PBLs than in BM (Figure 1A). These findings were confirmed on further examination of FOXP3 expression where 70% of the CD4/CD25 cells in normal marrow expressed FOXP3 compared with 30% in the peripheral blood of the same persons (Figure 1B). In contrast, myeloma patients showed only 2.2% of CD4+/CD25+ cells expressing FOXP3 in the BM compared with 52.2% in the peripheral blood (P < .001; Figure 1B). These data underscore unique differences in the BM microenvironment of normal hosts compared with myeloma patients. To further demonstrate whether these FOXP3+ cells behaved as bona fide Tregs, classic suppression assays were performed. We had previously demonstrated the potent tumor specificity of anti-CD3/CD28 aMILs.10 MILs were similarly activated and then either added directly to autologous CD138+ plasma cells alone (aMILs plus tumor) or in conjunction with unactivated autologous CD4+/CD25+ MILs or PBLs. Tumor specificity was determined by 3H-thymidine incorporation at 48 hours. As shown in Figure 1C, the addition of CD4+/CD25+-selected, unactivated MILs to aMILs did not affect their proliferative capabilities compared with aMILs alone. In contrast, the addition of unactivated CD4+/CD25+ PBLs resulted in greater than 90% suppression of T-cell proliferation. These data thus confirm that CD4+/CD25+ MILs in myeloma patients are not regulatory T cells.
MILs in myeloma patients contain few regulatory T cells. (A) CD25 expression. BM and peripheral blood from both myeloma patients and normal donors were stained for CD4 and CD25 expression. Mean fluorescent intensity for CD25 expression is indicated in parentheses in the right upper quadrant of each dot plot. (B) FOXP3+ expression on CD4+/CD25+ cells. Intracellular FOXP3 staining was performed on T cells. Graphed is the percentage of FOXP3 expression in the CD4+/CD25+ cells. Patients, N = 12; normal, N = 3. (C) Suppression assays with CD25+ cells. MILs from myeloma patients were stimulated with anti-CD3/CD28 beads alone, with the addition of unactivated CD4+/CD25+ autologous MILs at a 1:1 ratio of MILs to CD4+/CD25+ MILs, or with the addition of unactivated CD4/CD25 PBLs at the 1:1 ratio. After a 5-day stimulation, tumor-specific proliferation of MILs was determined by 3H-thymidine incorporation after 48 hours.
MILs in myeloma patients contain few regulatory T cells. (A) CD25 expression. BM and peripheral blood from both myeloma patients and normal donors were stained for CD4 and CD25 expression. Mean fluorescent intensity for CD25 expression is indicated in parentheses in the right upper quadrant of each dot plot. (B) FOXP3+ expression on CD4+/CD25+ cells. Intracellular FOXP3 staining was performed on T cells. Graphed is the percentage of FOXP3 expression in the CD4+/CD25+ cells. Patients, N = 12; normal, N = 3. (C) Suppression assays with CD25+ cells. MILs from myeloma patients were stimulated with anti-CD3/CD28 beads alone, with the addition of unactivated CD4+/CD25+ autologous MILs at a 1:1 ratio of MILs to CD4+/CD25+ MILs, or with the addition of unactivated CD4/CD25 PBLs at the 1:1 ratio. After a 5-day stimulation, tumor-specific proliferation of MILs was determined by 3H-thymidine incorporation after 48 hours.
The BM microenvironment in myeloma skews MILs toward an IL-17 phenotype
The results detailed in the previous paragraph left unanswered which mechanism(s) of tumor-induced immunosuppression might be operative in the BM and why myelomatous BM contains few Tregs compared with normal BM. Myeloma cells secrete a number of OC activating factors, including TGF-β, IL-6, RANKL, and MIP-1α.23,24 IL-6 in particular is critical in determining the fate of CD4 toward Th17 or Treg differentiation.15,25 In light of these findings and considering the contribution of myelomatous plasma cells on IL-6 producion, we examined the impact of the cytokine profile within the BM microenvironment on the T-cell profile. IL-6, TGF-β, and IL-1β skew T cells toward a Th17 phenotype, whereas IL-23 maintains and expands this subset. As expected, the IL-6 concentration was higher in myeloma BM plasma compared with normal BM plasma (29.6 pg/mL, range, 2-70 pg/mL; vs 3.7 pg/mL, range, 0-20 pg/mL) as shown in Table 1. IL-1β concentrations were also higher in myeloma BM plasma compared with normal BM plasma (29.9 pg/mL, range, 10.1-69.2 pg/mL; vs 5.8 pg/mL, range, 0-9.8 pg/mL) as were IL-23 levels: myeloma BM (246.4 pg/mL, range, 20-650 pg/mL; normal BM, 35.9 pg/mL, range, 28.0-44.0 pg/mL). No significant differences were appreciated in TGF-β levels (59.9 pg/mL myeloma BM vs 52.6 pg/mL normal BM). These data show that the cytokines necessary to skew and maintain a Th17 T-cell phenotype are found primarily in the BM compartment of myeloma patients compared with the blood and were absent in the BM of normal donors.
Cytokine analysis of myeloma and normal donors
| . | Bone marrow . | Peripheral blood . | ||
|---|---|---|---|---|
| Myeloma (N = 40) . | Normal (N = 12) . | Myeloma (N = 40) . | Normal (N = 12) . | |
| IL-6, pg/mL | 29.6 (2.0-70.2) | 3.7 (0-20.0) | 6.5 (4.4-8.0) | 2.3 (2.0-2.4) |
| TGF-β, pg/mL | 59.9 (20.8-80.0) | 52.6 (39.3-79.9) | 17.5 (1.7-39.4) | 16.1 (2.4-35.5) |
| IL-23, pg/mL | 246.4 (20.0-650.0) | 35.9 (28.0-44.0) | 19.6 (0-30.0) | 18 (11.0-24.0) |
| IL-1β, pg/mL | 29.9 (10.1-69.2) | 5.8 (0-9.2) | 4.5 (2.4-6.2) | 3.5 (0-4.9) |
| IL-17, pg/mL | 12.2 (2.2-35.0) | 5.1 (3.8-8.8) | 0.4 (0-2.2) | 0 (0-0) |
| MIP-1α, pg/mL | 1059.5 (98.2-4860.0) | 42.6 (38.4-62.8) | 14.8 (0-56.2) | 0 (0-0) |
| . | Bone marrow . | Peripheral blood . | ||
|---|---|---|---|---|
| Myeloma (N = 40) . | Normal (N = 12) . | Myeloma (N = 40) . | Normal (N = 12) . | |
| IL-6, pg/mL | 29.6 (2.0-70.2) | 3.7 (0-20.0) | 6.5 (4.4-8.0) | 2.3 (2.0-2.4) |
| TGF-β, pg/mL | 59.9 (20.8-80.0) | 52.6 (39.3-79.9) | 17.5 (1.7-39.4) | 16.1 (2.4-35.5) |
| IL-23, pg/mL | 246.4 (20.0-650.0) | 35.9 (28.0-44.0) | 19.6 (0-30.0) | 18 (11.0-24.0) |
| IL-1β, pg/mL | 29.9 (10.1-69.2) | 5.8 (0-9.2) | 4.5 (2.4-6.2) | 3.5 (0-4.9) |
| IL-17, pg/mL | 12.2 (2.2-35.0) | 5.1 (3.8-8.8) | 0.4 (0-2.2) | 0 (0-0) |
| MIP-1α, pg/mL | 1059.5 (98.2-4860.0) | 42.6 (38.4-62.8) | 14.8 (0-56.2) | 0 (0-0) |
To determine whether this cytokine profile correlated with an IL-17 phenotype in the myelomatous MILs, CD3+ cells from the blood and BM of myeloma patients and normal donors were stimulated for 4 hours with PMA and ionomycin. Intracellular staining was then performed on CD3+ cells for IL-17 and IFN-γ. As expected, normal MILs and PBLs primarily possessed a Th1 phenotype (Figure 2A). Among normal donors, the IL-17/ IFN-γ ratio in PBLs was 0.21 (IFN-γ production: 12.1% of CD3 cells) and in MILs was 0.12 (IFN-γ production: 10.7% of CD3 cells). In contrast, T cells from myeloma patients demonstrated a Th17 phenotype with an IL-17/IFN-γ ratio of 12.8 (IL-17 production: 5.5% of CD3 cells) for PBLs and 22.7 (IL-17 production: 24.3% of CD3 cells) for MILs. These data highlight the significant phenotypic differences between normal (Th1) and myelomatous (Th17) MILs, which are probably induced by the unique cytokine profile produced within the tumor microenvironment. To examine the complete extent of IL-17 expression among the MILs, we repeated the intracellular staining on CD4+ or CD8+ T cells from normal donors as well as myeloma patients with or without bone disease (Figure 2B). IFN-γ production is slightly reduced in myeloma patients without bone disease but markedly suppressed in patients with bone disease at the expense of IL-17 production. Interestingly, myeloma patients without bone disease possess a population of MILs that produce both IFN-γ+ and IL-17+ in both CD4 and CD8 T cells, whereas MILs from myeloma patients with bone disease produce almost exclusively IL-17 in both T-cell subsets.
Myeloma MILs produce IL-17. (A) Intracellular IL-17 staining. MILs and PBLs from either myeloma patients or normal donors were stimulated for 6 hours with PMA and ionomycin in the presence of Golgi-Stop. Cells were stained with CD3+, and intracellular staining was then performed for IL-17 and IFN-γ. Data are gated on CD3+ cells. (B) CD4/CD8/IL-17 staining. BM from myeloma patients with (n = 3) and without (n = 3) bone disease as well as from normal donors (n = 3) was stimulated for 6 hours with PMA and ionomycin in the presence of Golgi-Stop. Intracellular staining was performed for IL-17 and IFN-γ on either CD4 or CD8 T cells. Shown is the percentage of T cells expressing the respective cytokine. (C) Normal marrow plasma fails to induce a Th17 profile. Myeloma PBLs were cultured for 5 days in medium alone (no plasma), 50% normal BM plasma, or 50% normal BM plasma supplemented with the indicated cytokines at the following concentrations: IL-6, 10 ng/mL; TGF-β, 5 ng/mL; and IL-17, 10 ng/mL. After a 5-day incubation, the cells were stimulated for 6 hours with PMA and ionomycin and stained for CD3 and intracellular IL-17. Data are gated on CD3+ cells. (D) IL-17 produced by the myelomatous marrow microenvironment skews PBLs toward a Th17 profile. Myeloma PBLs were incubated for 5 days in the presence of medium alone, 50% myeloma BM plasma with or without anti–TGF-β 10 μg/mL, anti–IL-6 10 μg/mL, or both. Cells were cultured for 5 days as described in “Methods” and stained for intracellular IL-17.
Myeloma MILs produce IL-17. (A) Intracellular IL-17 staining. MILs and PBLs from either myeloma patients or normal donors were stimulated for 6 hours with PMA and ionomycin in the presence of Golgi-Stop. Cells were stained with CD3+, and intracellular staining was then performed for IL-17 and IFN-γ. Data are gated on CD3+ cells. (B) CD4/CD8/IL-17 staining. BM from myeloma patients with (n = 3) and without (n = 3) bone disease as well as from normal donors (n = 3) was stimulated for 6 hours with PMA and ionomycin in the presence of Golgi-Stop. Intracellular staining was performed for IL-17 and IFN-γ on either CD4 or CD8 T cells. Shown is the percentage of T cells expressing the respective cytokine. (C) Normal marrow plasma fails to induce a Th17 profile. Myeloma PBLs were cultured for 5 days in medium alone (no plasma), 50% normal BM plasma, or 50% normal BM plasma supplemented with the indicated cytokines at the following concentrations: IL-6, 10 ng/mL; TGF-β, 5 ng/mL; and IL-17, 10 ng/mL. After a 5-day incubation, the cells were stimulated for 6 hours with PMA and ionomycin and stained for CD3 and intracellular IL-17. Data are gated on CD3+ cells. (D) IL-17 produced by the myelomatous marrow microenvironment skews PBLs toward a Th17 profile. Myeloma PBLs were incubated for 5 days in the presence of medium alone, 50% myeloma BM plasma with or without anti–TGF-β 10 μg/mL, anti–IL-6 10 μg/mL, or both. Cells were cultured for 5 days as described in “Methods” and stained for intracellular IL-17.
To more accurately assess the impact of these cytokine on T-cell skewing, we examined the ability of exogenous cytokines to skew T cells toward a Th17 profile. Myeloma PBLs were cocultured with pooled BM plasma from normal donors with the addition of the respective cytokines as indicated (Figure 2C). Consistent with prior observations, plasma from normal donors failed to induce a Th17 profile. IL-6 in combination with TGF-β was critical to inducing the Th17 phenotype, whereas either cytokine administered alone had no effect. To confirm that the tumor microenvironment was, indeed, responsible for generating this population, the experiment was repeated using myeloma PBLs cocultured with pooled BM plasma from myeloma patients (Figure 2D). Myeloma BM plasma effectively converted myeloma PBLs to Th17 cells. Confirming the findings observed in Figure 2B, both IL-6 and TGF-β (and not each independently) were required for the generation of this phenotype as simultaneous blockade of both cytokines was required for abrogation of the Th17 phenotype. The requirement for both IL-6 and TGF-β to generate the Th17 phenotype speaks to the synergistic role these cytokines play at least in this system.
OC formation is mediated by IL-17–producing cells
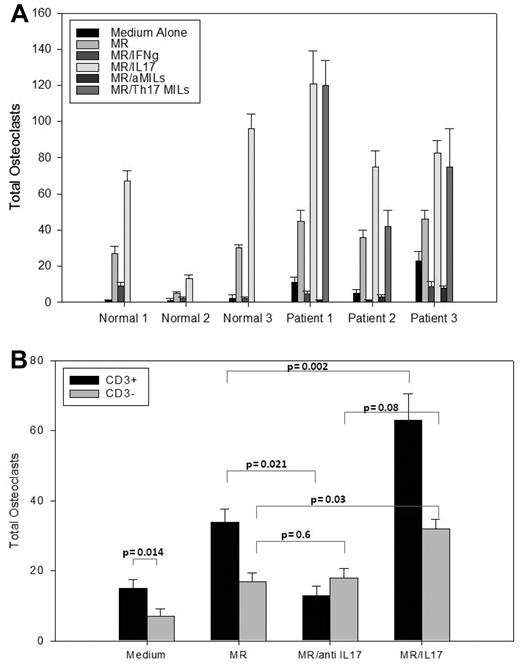
The aforementioned data point to IL-17–producing cells as the dominant T-cell population residing within the BM microenvironment in myeloma patients. However, the overall function of these effector T cells in myeloma remained unclear. Th17 cells have previously been described as playing a significant role in a variety of autoimmune phenomena, including the induction of lytic bone disease in rheumatoid arthritis.16 Considering the role of OCs in the lytic bone disease seen in myeloma, we examined the effect of IL-17 on OC formation. BM from myeloma patients and normal donors was cultured as described. Macrophage colony-stimulating factor and RANKL are typically required to induce OC formation. OC numbers were increased with the addition of these cytokines (Figure 3A). Interestingly, increased numbers of OCs were also observed in the myeloma BM containing no exogenous cytokines compared with the normal counterparts (14 vs 1.5), supporting the hypothesis that the endogenous cytokine milieu in the myelomatous microenvironment favors OC formation. The increase in OC formation with the addition of exogenous IL-17 in both normal and myeloma samples supports the role of IL-17 in OC formation. As expected, the addition of Th17-skewed MILs from myeloma patients (Th17 MILs) resulted in similar increases in OC formation.
IL-17–producing MILs increase osteoclastogenesis. (A) BM from normal donors and myeloma patients was incubated for 21 days in medium alone, macrophage colony-stimulating factor and RANKL (MR), MR plus IFN-γ, or MR plus IL-17. Myeloma BM was also incubated with MR plus autologous aMILs (Th1 skewed cells), or MR plus autologous Th17 skewed MILs. OC colonies were scored on day 21 by staining with 23-C6. Data are representative of 10 patients. (B) OC formation is T cell dependent. Ficolled BM from myeloma patients were either CD3 depleted or left unmanipulated and incubated for 21 days in medium alone or with the addition of MR, IL-17, MR/IL-17, MR plus anti–IL-17, or anti–IL-17. Cells were stained with 23-C6 and OCs were scored.
IL-17–producing MILs increase osteoclastogenesis. (A) BM from normal donors and myeloma patients was incubated for 21 days in medium alone, macrophage colony-stimulating factor and RANKL (MR), MR plus IFN-γ, or MR plus IL-17. Myeloma BM was also incubated with MR plus autologous aMILs (Th1 skewed cells), or MR plus autologous Th17 skewed MILs. OC colonies were scored on day 21 by staining with 23-C6. Data are representative of 10 patients. (B) OC formation is T cell dependent. Ficolled BM from myeloma patients were either CD3 depleted or left unmanipulated and incubated for 21 days in medium alone or with the addition of MR, IL-17, MR/IL-17, MR plus anti–IL-17, or anti–IL-17. Cells were stained with 23-C6 and OCs were scored.
In contrast to the enhancing effects of IL-17 in OC formation, BM cells cultured in the presence of IFN-γ significantly inhibited OC formation (Figure 3A, MR/IFN-γ). Previously, we showed that activation of MILs with anti-CD3/CD28 beads augments antimyeloma specificity by inducing a potent Th1 phenotype in myeloma patients.10 We thus cocultured OCs with IFN-γ–producing aMILs from myeloma patients. In such conditions, there was a significant reduction in OC formation, and the absolute number of OCs was less in this group than in the group cultured with medium alone. Taken together, our data implicate unactivated MILs as mediators of osteoclastogenesis in multiple myeloma. Furthermore, the ability of Th1-skewed activated MILs to impair and possibly reverse bone disease together with its previously reported antimyeloma activity underscores an additional potential therapeutic benefit of aMILs.
To determine whether IL-17–mediated increases in OC formation were T-cell dependent, the experiments were repeated in myeloma samples using either intact or T cell–depleted BM. As shown in Figure 3B, OC formation in the medium alone group was reduced in the 13 patients tested on average from 16 to 6 in the T cell–depleted group (P = .014). Exogenous IL-17 augmented OC numbers, but the effect was again diminished in the T cell–depleted groups. Importantly, IL-17 blockade significantly reduced OC formation in the intact BM (P = .021) but not the T cell–depleted group (P = .6) compared with no blockade. Taken together, these data provide evidence that: (1) the BM microenvironment in myeloma facilitates OC formation; and (2) OC formation is influenced by T cell–mediated IL-17 production.
The Th-17 cytokine profile in the marrow correlates with the extent of lytic bone disease
Next, we sought to determine whether a correlation existed between the Th17 phenotype and the extent of lytic bone disease in myeloma patients. We examined the extent of lytic bone disease in a cohort of 40 myeloma patients consisting of 26 untreated and 14 previously treated persons. Using a modified scoring system of lytic bone disease,19 a score of 0 was given to patients with no bone disease; 1, patients with one lesion measuring less than 1 cm; 2, patients with one lesion greater than 1 cm; 3, multiple lesion of less than 1 cm; and 4, multiple lesions greater than 1 cm. In addition to the cytokines involved in the Th17 cascade, MIP-1α, a chemokine produced by myeloma cells known to induce OC activity and to correlate with the severity of bone disease,26 was also measured. A statistically significant correlation between bone disease and MIP-1α plasma levels thus validated the scoring system (Figure 4A). Interestingly, the most significant statistical correlation between MIP-1α and bone disease occurred with advanced-stage bone disease (P = .00057) between multiple small lesions (grade 3) and multiple large lesions (grade 4).
The correlation between the Th17 marrow-derived cytokine profile and lytic bone disease in myeloma patients. (A) MIP-1α, TGF-β, IL-6, IL-1β, IL-23, and IL-17 levels were determined by ELISA on myeloma BM plasma. The correlation between cytokine levels and the extent of bone disease is graphed (n = 40). Values are plotted on a log2 scale. (B) Clinical parameters and bone disease. Serum calcium, alkaline phosphatase, and the percentage plasma cells in the BM were graphed in relation to the extent of bone disease in the patients (n = 40).
The correlation between the Th17 marrow-derived cytokine profile and lytic bone disease in myeloma patients. (A) MIP-1α, TGF-β, IL-6, IL-1β, IL-23, and IL-17 levels were determined by ELISA on myeloma BM plasma. The correlation between cytokine levels and the extent of bone disease is graphed (n = 40). Values are plotted on a log2 scale. (B) Clinical parameters and bone disease. Serum calcium, alkaline phosphatase, and the percentage plasma cells in the BM were graphed in relation to the extent of bone disease in the patients (n = 40).
Several cytokines involved in the generation of the Th17 profile were examined. The combination of TGF-β and IL-6 is known to drive naive T cells to the Th17 phenotype, whereas TGF-β expression in the absence of IL-6 induces a reciprocal development of Tregs.15 IL-1β increases the efficiency of Th17 differentiation,27 whereas IL-23 is required for the IL-17–mediated effector function and survival, but not differentiation.28 We thus examined the association between these cytokines and the extent of bone disease in our patient cohort as they relate to the Th17 profile. As shown in Figure 4A, BM plasma TGF-β levels did not correlate with bone disease, whereas IL-6 levels reached statistical significance, even in the early transition between grades 0 and 2 bone disease (P = .0009) and again between grades 3 and 4 (P = .00003). IL-1β levels were also significant in the cohorts demonstrating evidence of bone disease versus grade 0. However, its levels dramatically increased with advanced-stage bone disease (grade 3 vs grade 4, P < .00001). IL-23 cytokine levels did not correlate with the extent of lytic bone disease, although their levels exceeded those found in the plasma of normal BM by 8-fold (data not shown). Lastly, IL-17 BM plasma levels directly correlated with the extent of bone disease, especially for the transition between single to multiple bone lesions.
We also examined whether a correlation existed between clinical parameters and bone disease. As shown in Figure 4B, the only clinical factor demonstrating a direct correlation with bone disease was the alkaline phosphatase levels (a marker of bone turnover). Ethnicity, International Staging System stage III disease (which considers albumin and β-2 microglobulin levels), immunoglobulin subtype, or poor-risk chromosomal translocations (del 13, t(4;14), t(14;16), or del 17) failed to correlate with the extent of bone disease (data not shown). Surprisingly, the actual tumor burden, as measured by the percentage plasma cells in the BM biopsies, also failed to correlate with either the extent of lytic bone disease or the Th17 cytokine profile described above. Taken together, these findings suggest that the lytic bone disease is mediated by the local inflammatory response independent of the tumor burden.
Discussion
This study offers several new insights regarding the interaction of the immune system and bone metabolism within the tumor microenvironment in myeloma. Specifically, in contrast to normal BM where Tregs represent the major component of the CD4+/CD25+ population, there is a notable absence of Tregs in the myeloma microenvironment and an increase of IL-17–producing T cells. This difference is probably explained by the myeloma cell-mediated IL-6 production. We demonstrated that IL-17–producing T cells induce OC activation and that IL-17 production directly correlates with lytic bone disease irrespective of the tumor burden. Taken together, these findings underscore that bone destruction in myeloma is not a direct result of the tumor burden but rather is tightly dependent on the tumor-induced immune system within the BM microenvironment. As such, we propose that reversal of the IL-17 phenotype potentially provides a novel strategy for targeting OC-mediated bone destruction in myeloma.
The myeloma microenvironment is a tightly regulated network of interactions between stroma elements, malignant plasma cells, and the immune system culminating in an increase in IL-6 production. Indeed, analysis of the BM cytokines revealed significantly higher IL-6 levels in myeloma compared with normal BM plasma, whereas levels of TGF-β remained unchanged (Table 1). This combination of IL-6 and TGF-β can generate Th17-producing T cells with a reciprocal reduction in Tregs.15 These findings raise a couple of questions:
What is the role of Tregs in myeloma and do they correlate with disease burden in a manner similar to what has previously been described for solid tumors?
Are the “effector” IL-17 cells exerting an antitumor or pathologic effect?
Considering the predominance of Th17 in advanced disease and the relative absence of Tregs in the BM, Tregs probably represent a small component of the immunosuppressive pathways accompanying this disease and may, indeed, define a desired steady state present in the normal BM. This is supported by the predominance of Tregs in normal BM9 and accumulation of Tregs in myeloma patients after allogeneic BM transplantation.29 In contrast, we show that advanced-stage myeloma is associated with a Th17 T-cell phenotype and a reciprocal reduction in Tregs, thereby establishing a role for the IL-17–producing cells in the malignant phenotype of myeloma.
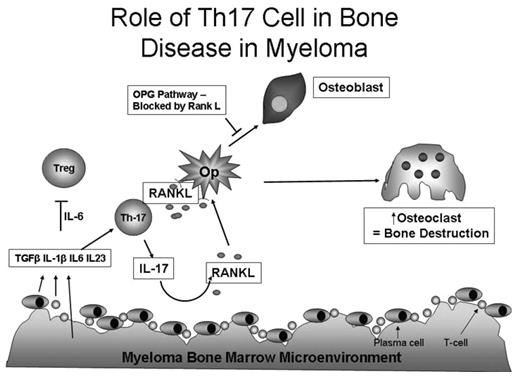
Our data underscore the critical role of the IL-17 pathway in generating osteolytic bone lesions in myeloma. This most probably occurs through a coordinated interplay among various components within the myelomatous BM microenvironment that maintain conditions favoring tumor growth as well as bone destruction. Myeloma cells may be able to directly destroy bone,30 and OCs can possess chromosomal abnormalities present in primary myeloma clones suggesting a fusion of these 2 cells.31 Despite evidence of this direct tumor-bone interaction, we think that T cells play a critical role in this process as proposed in Figure 5. A critical cytokine in this process is IL-6. Its production by stromal mesenchymal stem cells and possibly dendritic cells augments myeloma plasma cell survival.32 IL-6 production by either the tumor or stromal environment, possibly in conjunction with IL-7, has been implicated in RANKL up-regulation and IFN-γ down-regulation in T cells with the subsequent increase in OC formation.33 We now propose that IL-6 and IL-23 in combination with TGF-β activate retinoic acid-related orphan receptorγt together with STAT3 to generate the Th-17 phenotype,14 which in turn mediates the T cell-induced lytic bone disease. It also appears that there is a contribution of IL-17–producing CD8+ cells (Tc17 cells), which possess reduced cytotoxicity,34 although its precise role in bone disease remains to be determined.
Proposed model of the role of IL-17–producing MILs in myeloma bone disease. The tumor microenvironment, consisting of plasma cells, stromal elements, and MILs, produces IL-6, TGF-β, IL-1β, and IL-23. The excess of IL-6 compared with TGF-β causes a shift from a Treg (predominantly found in a normal BM) to a Th17 cell, which in turn increases T-cell production of IL-17 and RANKL. These 2 cytokines together with MIP-1α subsequently act on OC precursors (Op) to increase osteoclastogenesis. The addition of IFN-γ–producing T cells inhibits osteoclastogenesis.
Proposed model of the role of IL-17–producing MILs in myeloma bone disease. The tumor microenvironment, consisting of plasma cells, stromal elements, and MILs, produces IL-6, TGF-β, IL-1β, and IL-23. The excess of IL-6 compared with TGF-β causes a shift from a Treg (predominantly found in a normal BM) to a Th17 cell, which in turn increases T-cell production of IL-17 and RANKL. These 2 cytokines together with MIP-1α subsequently act on OC precursors (Op) to increase osteoclastogenesis. The addition of IFN-γ–producing T cells inhibits osteoclastogenesis.
Support of this hypothesis comes from 2 pieces of data presented here. First, as shown in Figure 3, OC formation is significantly augmented by the exogenous administration of IL-17, in the presence of IL-17–producing MILs, or with BM plasma from myeloma patients. Conversely, OC formation in the presence of IL-17 blockade is dramatically diminished in a T cell-depleted BM. Second, clinically we show a highly statistically significant correlation between lytic bone disease and the Th17 phenotype in the BM of myeloma patients. Furthermore, the absence of any correlation with other parameters, such as tumor burden or myeloma subtype, underscores the dominant role of the immune response in mediating bone destruction. As such, strategies targeting this pathway could have significant clinical benefit.
These findings suggest 3 potential approaches that could be used to reduce OC formation. First, as shown in Figure 1B, the marrow of normal persons possesses few Th17 but shows a predominance of Tregs. CTLA-4 is up-regulated on Tregs.35 Furthermore, CTLA-4 directly binds to OC precursors to suppress osteoclastogenesis.36 Potentially, the reduction in numbers of Tregs in patients with myeloma could contribute to lytic bone disease. Conversely, either homeostatic restoration of the normal BM environment (increases in Tregs) or the therapeutic use of CTLA-4-Ig could be used to reduce bone destruction. Second, IFN-γ–producing Th1 T cells block the differentiation and maturation of OCs. aMILs effectively switch from Th17 to the IFN-γ–producing, Th1 phenotype. As such, aMILs may be critical in preventing further progression of lytic bone disease. Third, the Th17 phenotype depends on STAT3 activation as the critical transcriptional regulator of retinoic acid-related orphan receptor γt and IL-17 itself.37-39 Pharmacologic strategies aimed at disrupting this pathway could prove beneficial in reducing bone disease. Bisphosphonates are the mainstay of therapy in delaying myeloma bone disease. Interestingly, the nitrogen-containing compounds, such as pamidronate and zoledronate, are known to activate γδ T cells,40 which in turn can be major producers of IL-17.41 As such, blocking IL-17 production could synergize with bisphosphonate therapy. Of the known pharmacologic therapies used in the treatment of myeloma, both lenalidomide and bortezomib inhibit OC activation through down-regulation of MIP-1α, BAFF, and APRIL.42 Interestingly, we recently demonstrated that the decrease in tumor burden in lenalidomide-treated patients is also associated with a decrease in IL-17 production by MILs (K.N. et al, manuscript submitted).
In conclusion, our data demonstrate that the Th17 T-cell phenotype is a key predictor of lytic bone disease in multiple myeloma. The highly significant statistical relationship between the Th17 immune phenotype in the marrow and OC formation strongly supports the rationale for developing therapeutic strategies aimed at the immune regulation of osteoclastogenesis as an approach to reduce or prevent progression of bone disease in multiple myeloma.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank the Ercole Comini Charity for their support, without which most of this work would not have been possible.
Authorship
Contribution: K.N. designed and performed experiments, analyzed data, and contributed to manuscript preparation; L.M. performed statistical analysis of data; J.A. performed experiments; D.P. designed experiments and contributed to manuscript writing; G.D.R. designed experiments and contributed to manuscript writing; and I.B. designed experiments, analyzed data, and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ivan Borrello, 1650 Orleans St, CRB-1, Rm 453, Baltimore, MD 21231; e-mail: iborrell@jhmi.edu.