Abstract
Secondary lymphoid organs provide a unique microenvironment for generation of immune responses. Using a cell type–specific conditional knockout approach, we have dissected contributions of tumor necrosis factor (TNF) produced by B cells (B-TNF) or T cells (T-TNF) to the genesis and homeostatic organization of secondary lymphoid organs. In spleen, lymph nodes and Peyer patches, the cellular source of TNF, and its molecular form (soluble versus membrane-bound) appeared distinct. In spleen, in addition to major B-TNF signal, a complementary T-TNF signal contributed to the microstructure. In contrast, B-TNF predominantly controlled the development of follicular dendritic cells and B-cell follicles in Peyer patches. In lymph nodes, cooperation between TNF expressed by B and T cells was necessary for the maintenance of microarchitecture and for generation of an efficient humoral immune response. Unexpectedly, soluble but not membrane TNF expressed by B cells was essential for the organization of the secondary lymphoid organs. Thus, the maintenance of each type of secondary lymphoid organ is orchestrated by distinct contributions of membrane-bound and soluble TNF produced by B and T lymphocytes.
Introduction
Secondary lymphoid organs provide an important microenvironment for the initiation and development of efficient immune responses. Their highly sophisticated structure allows migration and interactions between antigen-presenting cells, T and B lymphocytes, as well as follicular dendritic cells (FDCs) and other stromal cells. The cooperation of the lymphoid cells within secondary lymphoid organs dramatically increases the probability of interactions of rare B, T, and antigen-presenting cells that result in effective generation of humoral immune responses (reviewed in Fu and Chaplin,1 Mebius,2 and Allen et al3 ).
Tumor necrosis factor (TNF) and lymphotoxin (LT) are cytokines required for both formation and maintenance of the microarchitecture of the secondary lymphoid organs, acting primarily through their receptors TNFRp55 and LTβR, respectively, and engaging classical and alternative nuclear factor-κB (NF-κB) pathways.1,4,5 In vivo TNF is produced by many cell types, including lymphoid and stromal cells, and can exist in membrane-bound as well as in soluble forms.6 Systemic TNF ablation in mice results in the impairment of humoral immune responses, host defense functions, and in multiple defects in lymphoid tissues including disruption of primary B-cell follicles and absence of germinal centers (GCs) and FDCs.6-10
FDCs are key components in the dynamic organization of the germinal center structure and are essential for generation of efficient immune responses as well as for support of follicular microarchitecture and migration of B cells to the follicles.3,11-13 Accordingly, mice that lack FDCs show reduced specific immunoglobulin G (IgG) antibody responses to T-cell–dependent antigens.1,5
Several studies addressed TNF- and LT-dependent mechanisms that may regulate the generation of FDCs and B-cell follicles in different secondary lymphoid organs.14,15 In particular, in contrast to the spleen, the generation of FDCs in lymph nodes (LNs) and PP is independent of surface LT expression by B and T cells.4,15 While the critical role of B-cell–derived TNF and LT for development of FDCs and B-cell follicles in spleen has been well established,1,16,17 the contribution of various TNF-producing cells in organization of secondary lymphoid organs other than spleen remains unknown.
To define the role of TNF produced by specific cell types in development and maintenance of secondary lymphoid organs, we used mice with conditional inactivation of TNF gene restricted to either B cells (B-TNF knockout [KO]) or T cells (T-TNF KO) or to both T cells plus B cells (T,B-TNF KO). Some of these mice were also crossed to mutant mice expressing only membrane-bound TNF18 to distinguish between 2 molecular forms of TNF produced by a given cellular source. Our results obtained using this experimental panel unravels distinct contributions of TNF signals originating from B and T cells to the maintenance of distinct lymphoid tissues, such as spleen, LNs, and Peyer patches (PPs), and to the efficiency of humoral immune responses to thymus-dependent antigens.
Methods
Mice
TNF-, T-TNF–, and B-TNF–deficient mice were genotyped as described.10,19 In addition, T-TNF KO mice were generated by crossing TNF floxed mice19 with lck-Cre transgenic mice.20 We found no difference in phenotypes of T-TNF KO based on lck-Cre deleter20 or CD4-Cre transgene.19 T,B-TNF KO mice were generated by intercrossing B-TNF and T-TNF KO mice. Analysis of TNF gene deletion and of the ablation of TNF production in this panel of mutant mice confirmed that TNF ablation occurred with high efficiency and specificity in the targeted cell lineages19 (and data not shown). Mice expressing only membrane form of TNF specifically in B or T cells (Tm-B-TNF KO and Tm-T-TNF KO mice) were generated by crossing B-TNF KO or T-TNF KO mice to knock-in mice expressing only membrane form of TNF.18 All mice were backcrossed at least 4 times to C57B6 background. For experiments, mice were housed under specific pathogen–free conditions. Animal care was provided in accordance with the procedures outlined in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals21 and to Russian and German regulatory requirements.
Immunohistochemistry
Immunohistochemistry was performed on 6-μm frozen sections as previously described.15 Processing and cryosectioning of tissues from multiple TNF conditional knockout mice were done in a single OCT block, and 2 serial sections per slide were stained to avoid processing artifacts. All antibodies were from BD Biosciences unless indicated otherwise. MOMA-1 and ER-TR7 antibodies were from Serotec. Sections were counterstained with Mayer hematoxylin, mounted with glycerol-gelatin (Dako), and documented using Zeiss Axiocam microscope equipped with Zeiss digital camera. Routinely, 10 different areas were photographed per section. Three slides per each mouse genotype (n = 5 mice in group from 3 independent experiments) were stained and quantified. Ten independent white pulp areas or T/B cell areas were quantified for each mouse per section. The size of GCs, FDC networks, and the marginal zone (MZ) was quantified using NIH ImageJ software Version 1.42 (http://rsbweb.nih.gov/ij/). A lasso tool was used to mark GC and FDC positive areas, and pixel area counts were recorded. After statistical evaluation, mean values with ranges for each genotype were selected for display purposes.
Measurements of thymus-dependent humoral response
Six- to 8-week-old mice were challenged intraperitoneally with 200 μL phosphate-buffered saline containing 108 sheep red blood cells (SRBCs; Cedarline Lab). Spleens and LN were prepared for immunohistochemistry 8 days later. Blood was taken from the retro-orbital sinus on day 21 after primary immunization. Specific antibodies were measured and analyzed as previously described.22 Splenectomy was done under aseptic conditions according to the NIH guidelines. To measure the effect of anti-TNF therapy on antibody production, mice were challenged intraperitoneally with 200 μL of phosphate-buffered saline or Etanercept (30 mg/kg) twice per week, and 108 SRBCs were injected on day 7. Blood was taken from the tail vein on day 21 postprimary immunization. Specific antibodies were measured and analyzed as previously described.22
Gene expression profiling
RNA was isolated from mesenterial LNs of naive animals using TRI-Reagent (Sigma-Aldrich) and further purified with QIAGEN RNeasy minicolumns. Cyanine-3–labeled cRNA was hybridized to the 44 000-feature 60-mer oligo microarray analysis.23 Triplicate data were analyzed, false discovery rate was set to ≤ 0.1, and genes with fold difference < 1.5 were excluded from significant gene lists. Array data were deposited into Gene Expression Omnibus (GEO; National Center for Biotechnology Information) under accession number GSE8610.
Real-time PCR analysis
RNA from cells or frozen tissues was isolated using RNeasy Mini kit (QIAGEN). Total RNA (2-5 μg) was treated with DNAse I (Ambion) and reverse-transcribed using Superscript III kit (Invitrogen). For SYBR-Green real-time polymerase chain reaction (PCR), we used 2 × Dynamo PCR Master Mix (FinEzyme). The concentration of the target gene was determined using comparative cycle threshold (CT; cycle threshold number at a cross-point between amplification plot and threshold) method and normalized to hypoxantine phosphoribosyltransferase or glyceraldehyde-3-phosphate dehydrogenase mRNA expression. For primer sequences, see supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical analysis
Comparisons of data were analyzed by 2-tailed Student t test using GraphPad Prism 5.0 program. P values less than .05 were considered significant.
Results
Maintenance of spleen microarchitecture is dependent primarily on B-cell–derived TNF with a distinct contribution of TNF produced by T cells
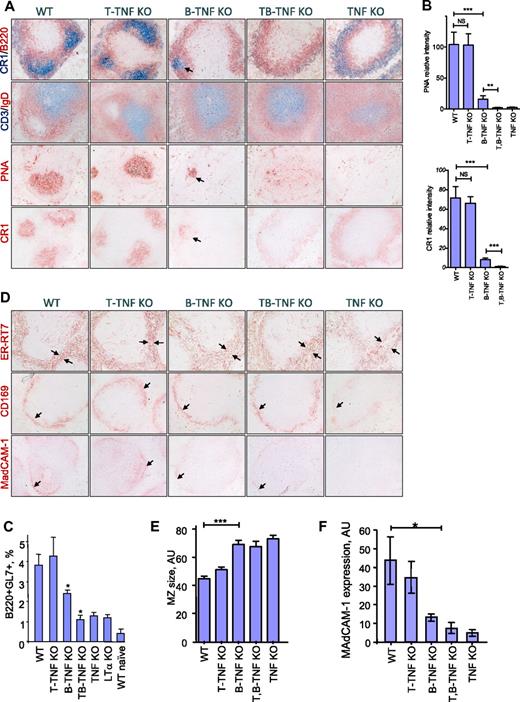
To assess the role of distinct cellular sources of TNF in the generation of splenic B-cell follicles, GCs, FDCs, and spleens from wild-type (WT) TNF floxed mice, TNF KO, T-TNF KO, B-TNF KO, and T,B-TNF KO mice were examined by immunohistochemistry after immunization with SRBCs, a T cell–dependent antigen. Mice were analyzed 8 days later, at peak of GC development.24 Considering previous data based on cell transfers into immunodeficient hosts,1,16,17,22 we anticipated that TNF from B cells would play an exclusive role in the formation of GCs and FDCs in the spleen. Indeed, the formation of B-cell follicles and GCs, as well as the development of FDC networks, were markedly impaired in spleens of B-TNF KO mice (Figure 1A-B). Nevertheless, these defects in spleen architecture in B-TNF KO mice were less severe than in mice with complete TNF deficiency. In particular, FDC clusters coinciding with IgD-negative areas within peanut agglutinin (PNA)–positive regions of GCs, although largely reduced compared with WT mice, could still be detected in spleens of B-TNF KO mice but not in TNF KO mice (Figure 1A-B). Aberrant FDC-M2 and CR1 staining was observed in the MZ of T,B-TNF KO mice and TNF KO mice, probably representing immature FDC precursors.25 The number of GC GL7+ B cells induced by SRBCs was partially reduced in B-TNF KO mice, compared with WT and TNF KO mice, reflecting the impaired development of GCs in these mice (Figure 1C). This phenotype of B-TNF KO mice was not due to abnormal numbers of B or T cells in the spleen (supplemental Figure 1A).
Maintenance of spleen microarchitecture is primarily dependent on TNF expressed by B cells with distinct contribution of T-cell–derived TNF. (A) Mice were immunized with i.p. SRBCs and spleen sections analyzed on day 8. B-TNF KO mice display reduced GCs, lack of polarized B-cell follicles, and disruption of FDC networks. Note that some FDC- and PNA-positive cells are present in the spleens of B-TNF KO mice (shown by arrows), while no FDCs and no polarized B-cell follicles are observed in spleen of T,B-TNF KO mice. Original magnification is ×200. Representative images from 1 of 3 independent experiments (n = 5 mice per group) are shown. (B) Quantification of GC and FDC areas from panel A. Data represent means ± SEM; *P < .05, **P < .01, ***P < .001. (C) Analysis of germinal center B cells. Mice were immunized with i.p. SRBCs, and GL7+ GC B cells were analyzed by flow cytometry on day 8 (represents 1 of 2 independent experiments, 3 mice per group). Data represent means ± SD; *P < .05. (D) MZ structure is dependent on TNF produced by B, T, and other cell types. Mice were immunized with i.p. SRBCs, and spleen sections were analyzed on day 8. Frozen spleen sections were labeled with anti-ER-TR7, anti-CD169, and anti-MAdCAM-1 antibodies. Note the increased breadth of the MZ (ER-TR7 labeling), reduced and disorganized MAdCAM-1, and CD169 staining in B-TNF, T,B-TNF, and TNF KO mice (marked by black arrows). Microphotographs were taken with an Olympus BX41 microscope with a Zeiss Axiocam digital camera; images were captured with Zeiss Imaging AxioVision (Version 4.7) and processed using Adobe Photoshop CS3 (Version 10.0). Original magnification is ×200. Representative images from 1 of 3 independent experiments (n = 5 mice per group) are shown. (E) Quantification of MZ size on panel D (ER-TR7 labeling). MZ size (shown by arrows) was quantified using NIH ImageJ software. Data represent means ± SEM. (F) Expression of MAdCAM-1 in spleens of indicated mutant mice measured by real-time PCR. Data represent means ± SD; n = 3, *P < .05.
Maintenance of spleen microarchitecture is primarily dependent on TNF expressed by B cells with distinct contribution of T-cell–derived TNF. (A) Mice were immunized with i.p. SRBCs and spleen sections analyzed on day 8. B-TNF KO mice display reduced GCs, lack of polarized B-cell follicles, and disruption of FDC networks. Note that some FDC- and PNA-positive cells are present in the spleens of B-TNF KO mice (shown by arrows), while no FDCs and no polarized B-cell follicles are observed in spleen of T,B-TNF KO mice. Original magnification is ×200. Representative images from 1 of 3 independent experiments (n = 5 mice per group) are shown. (B) Quantification of GC and FDC areas from panel A. Data represent means ± SEM; *P < .05, **P < .01, ***P < .001. (C) Analysis of germinal center B cells. Mice were immunized with i.p. SRBCs, and GL7+ GC B cells were analyzed by flow cytometry on day 8 (represents 1 of 2 independent experiments, 3 mice per group). Data represent means ± SD; *P < .05. (D) MZ structure is dependent on TNF produced by B, T, and other cell types. Mice were immunized with i.p. SRBCs, and spleen sections were analyzed on day 8. Frozen spleen sections were labeled with anti-ER-TR7, anti-CD169, and anti-MAdCAM-1 antibodies. Note the increased breadth of the MZ (ER-TR7 labeling), reduced and disorganized MAdCAM-1, and CD169 staining in B-TNF, T,B-TNF, and TNF KO mice (marked by black arrows). Microphotographs were taken with an Olympus BX41 microscope with a Zeiss Axiocam digital camera; images were captured with Zeiss Imaging AxioVision (Version 4.7) and processed using Adobe Photoshop CS3 (Version 10.0). Original magnification is ×200. Representative images from 1 of 3 independent experiments (n = 5 mice per group) are shown. (E) Quantification of MZ size on panel D (ER-TR7 labeling). MZ size (shown by arrows) was quantified using NIH ImageJ software. Data represent means ± SEM. (F) Expression of MAdCAM-1 in spleens of indicated mutant mice measured by real-time PCR. Data represent means ± SD; n = 3, *P < .05.
On the contrary, inactivation of TNF only in T cells (T-TNF KO mice) did not lead to any striking abnormalities in splenic microstructure (Figure 1A-B). However, simultaneous ablation of TNF in both T and B cells (T,B-TNF KO mice) resulted in a more severe disruption of the spleen microarchitecture, compared with B-TNF KO mice; FDC clusters were completely absent and no organized B-cell follicles and GCs could be found (Figure 1A-C). Analysis of spleen structure in naive mice with conditional TNF deficiency produced similar results (supplemental Figure 2), suggesting a role for TNF both in homeostatic conditions and during immune responses. B-cells follicles and FDCs were severely reduced in B-TNF KO mice, and additional inactivation of TNF from T cells in T,B-TNF KO mice resulted in complete disruption of B-cell follicles and lack of FDCs (supplemental Figure 2). The difference between spleen phenotypes of naive T-TNF KO and B-TNF KO mice was not due to reduced numbers of T or B cells in B-TNF KO mice because relative and absolute cells number of T and B cells were similar in all mutant mice analyzed (supplemental Figure 1). We found similar TNF expression in sorted T and B cells from the spleen and LNs (supplemental Figure 4), and observed a decrease in splenic TNF mRNA in B-TNF KO mice (supplemental Figure 5) corresponding to the prevalence of the B-cell over T-cell number in the spleen (supplemental Figure 1).
MZ structure is dependent on TNF produced by both B and T cells, as well as by other cell types
The MZ of the spleen serves is the first barrier to blood-borne pathogens and viruses.26,27 MZ structure is impaired in mice with compete TNF deficiency25 and in B-cell–deficient mice.28 Analysis of mice with conditional inactivation of TNF in T and B cells revealed that maintenance of the MZ was largely dependent on TNF produced by B cells, while TNF from T cells and other yet unknown cell types provided a complementary signal (Figures 1D-F and supplemental Figure 3). Immunolabeling of stromal components with anti-ER-TR7 antibody revealed an increased breadth of the MZ in B-TNF, T,B-TNF, and TNF KO mice. The layer of CD169 (MOMA-1) positive MZ metallophilic macrophages was strongly disorganized, while expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on sinus lining cells was gradually reduced in B-TNF, T,B-TNF, and TNF KO mice (Figure 1D-F and supplemental Figure 3). Overall, these results indicate that TNF produced not only by B and T cells but also by additional yet unknown cells is actively involved in the organization of the MZ structure.
Organization of LNs is dependent on cooperation between TNF-mediated signals originating from both B and T cells
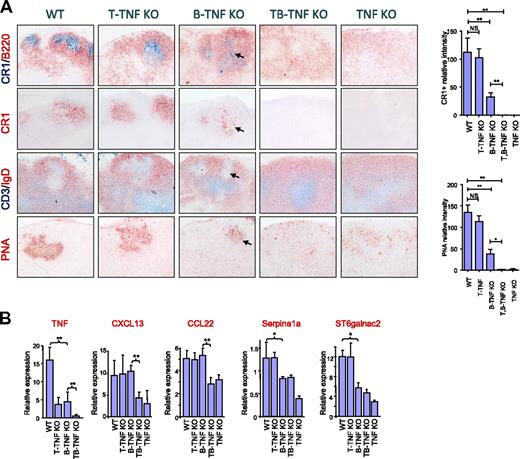
Because some cooperation between B-TNF and T-TNF was found in spleen, we next assessed the maintenance of LN microarchitecture in B-TNF, T-TNF, and T,B-TNF KO mice. Staining the mesenteric LNs (MLNs) with FDC-specific antibodies revealed reduced numbers of FDCs in B-TNF KO mice compared with WT mice (Figure 2A). B-TNF KO mice were able to develop GCs and B-cell follicles, which, however, displayed a less organized structure compared with LNs of WT mice. In particular, less defined GCs with reduced areas of PNA+ clusters and IgD negative areas were observed (Figure 2A). Moreover, reduced numbers of FDCs correlated with defective formation of GCs and B-cell follicles in MLNs of B-TNF KO mice. Surprisingly, MLNs in the double-deficient T,B-TNF KO mice completely lacked FDCs, failed to develop B-cell follicles and GCs, and showed a disturbed separation of T- and B-cell zones (Figure 2A). Thus, our data suggest that TNF from T cells can cooperate with B cell–derived TNF in the maintenance of MLN microarchitecture, including generation of FDCs and B-cell follicles.
Role of TNF produced by lymphocytes in supporting organization of LNs. (A) B and T cells cooperate by TNF production in generation of FDCs in LNs. Mice were immunized i.p. with 108 SRBCs and analyzed on day 8. Frozen sections of MLNs were stained with indicated antibodies. Magnification is ×200. Note the reduction of FDCs and GCs in both B-TNF KO mice (shown by arrows) and the complete absence of FDCs and GCs in T,B-TNF and TNF KO mice. Representative images from 1 of 3 independent experiments (n = 5 mice per group) are shown. Right panel shows quantification of CR1- and PNA-positive areas. Data represent means ± SEM; *P < .05, **P < .01. (B) TNF from B and T cells acts in concert in regulating TNF-dependent genes in LNs. Analysis of TNF and TNF-dependent gene expression in MLNs of indicated mutant mice (represents 1 of 2 independent experiments, n = 3 mice per group, means ± SD); *P < .05, **P < .01.
Role of TNF produced by lymphocytes in supporting organization of LNs. (A) B and T cells cooperate by TNF production in generation of FDCs in LNs. Mice were immunized i.p. with 108 SRBCs and analyzed on day 8. Frozen sections of MLNs were stained with indicated antibodies. Magnification is ×200. Note the reduction of FDCs and GCs in both B-TNF KO mice (shown by arrows) and the complete absence of FDCs and GCs in T,B-TNF and TNF KO mice. Representative images from 1 of 3 independent experiments (n = 5 mice per group) are shown. Right panel shows quantification of CR1- and PNA-positive areas. Data represent means ± SEM; *P < .05, **P < .01. (B) TNF from B and T cells acts in concert in regulating TNF-dependent genes in LNs. Analysis of TNF and TNF-dependent gene expression in MLNs of indicated mutant mice (represents 1 of 2 independent experiments, n = 3 mice per group, means ± SD); *P < .05, **P < .01.
In contrast to MLNs, the organization of PPs was predominantly regulated by B cell–derived TNF (supplemental Figure 6), consistent with a previous study.29 PP from B-TNF KO mice lacked FDCs and organized B-cell follicles (supplemental Figure 6). TNF produced by T cells did not play any appreciable role in organization of PPs since T-TNF KO mice displayed normal PP microarchitecture (supplemental Figure 6), and T,B-TNF KO mice did not show additional defects in PP structure, compared with B-TNF KO mice (supplemental Figure 6). Thus, TNF produced predominantly by B cells is necessary for the maintenance of PP microstructure.
TNF produced by both B and T cells is acting in concert to regulate expression of TNF- dependent genes in LNs
To define a relative contribution of B or T cells to overall TNF expression in LNs, we measured TNF mRNA levels in LNs of naive conditional TNF-deficient mice. Interestingly, in contrast to the spleen, in which TNF expression was mostly attributed to B cells (supplemental Figure 5), in LNs a significant amount of TNF was produced by T cells (Figure 2B). This difference may therefore be the reason for substantial contribution of T cells to the maintenance of LN structure. The relative per cell TNF expression levels, however, were comparable in splenic and LN T and B cells, ruling out an intrinsic difference in TNF expression between T or B cells in spleen and LNs (supplemental Figure 4).
To further define specific TNF-dependent transcriptional signatures that may help to understand the underlying mechanisms of cooperation between TNF from B and T cells in the maintenance of LN structure, we performed a microarray analysis of RNA expression from LNs of B-TNF and T,B-TNF KO mice. One of the genes down-regulated in LNs from T,B-TNF KO mice, compared with B-TNF KO mice, encoded the chemokine CXCL13 (BLC; Figure 2B), which is involved in navigation of B and activated T cells to the lymphoid follicles.30 Mice with deficiency of CXCL13 or its receptor CXCR5 have a very similar spleen phenotype as the T,B-TNF KO mice, with disruption of B-cell follicles and FDCs.30 In addition, macrophage-derived chemokine (MDC; CCL22) was significantly down-regulated in LNs of T,B-TNF KO mice, compared with B-TNF KO mice (Figure 2B). Consistently, both CXCL13 and CCL22 were previously reported to be expressed by FDCs.31 Thus, the reduced expression of these chemokines in T,B-TNF KO mice, compared with B-TNF KO mice, correlates with the complete lack of FDC clusters in LNs of T,B-TNF KO mice (Figure 2B).
We next examined the expression of several other genes known to be involved in the organization of the LN, such as Serpina1a and ST6Galnac2.32 Expression of Serpina1a, a serine peptidase inhibitor, was previously found to be FDC-specific.31 Sialyltransferase ST6Galnac2 is expressed by high endothelial venules (HEV) and is a marker of the HEV status.32 In contrast to CXCL13 and ST6Galnac2, the expression of Serpina1a was only partially reduced in LN of B-TNF KO mice and was further down-regulated in TNF-deficient mice, but appeared normal in T-TNF KO mice (Figure 2B). Thus, our data suggest that TNF produced by B and T cells are required for the maintenance of the integrity of LNs and act in concert in regulation of expression of CXCL13 and CCL22 genes.
Cooperation of TNF from both B and T cells is required for mounting an efficient humoral immune response
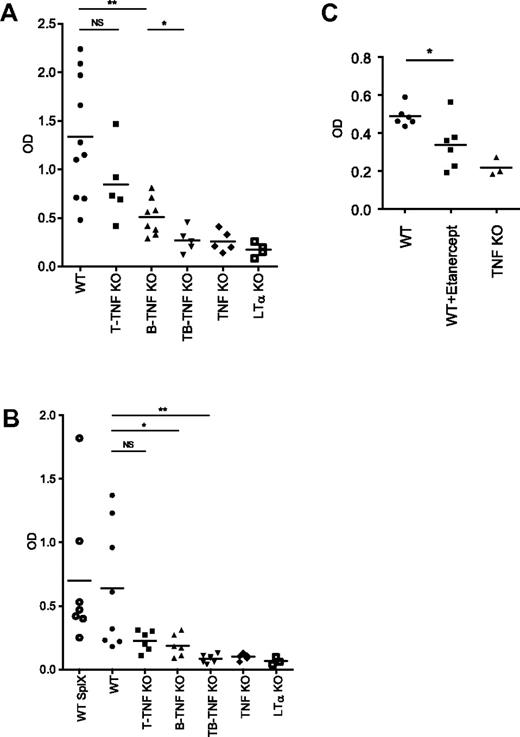
To define whether structural abnormalities of the secondary lymphoid organs in mice with lymphocyte-specific TNF deficiency translated into functional deficiencies, we immunized mice with SRBCs, a corpuscular antigen, which requires T cells help to induce an antibody response. Specific IgG responses were slightly reduced in both B-TNF and T-TNF KO mice, but not as severely as in mice with complete TNF deficiency, after intraperitoneal (i.p.) immunization (Figure 3A). In contrast, specific IgG levels in T,B-TNF KO mice were greatly reduced, similarly to TNF KO mice (Figure 3A). Interestingly, T-TNF KO mice displayed apparently normal spleen microarchitecture (Figure 1A), yet the IgG response to SRBCs was slightly reduced after i.p. immunization (Figure 3A), suggesting an insufficient immune priming in LNs. Consistently, splenectomized WT mice were able to generate substantial IgG response after i.p. immunization (supplemental Figure 7). Rechallenge of B-TNF KO mice 2 months after primary immunization partially restored IgG production (supplemental Figure 8), suggesting that generation of memory response is not impaired in these mice.
Inactivation of TNF production by both B and T cells results in impaired humoral response to T-cell–dependent antigen. (A) Mice were immunized i.p. with 108 SRBCs. Sera were collected on day 21, and anti-SRBC response was measured by ELISA. Symbols indicate individual mice. Mean values are indicated. IgG response is reduced both in B-TNF and T-TNF KO mice, however not as severe as in T,B-TNF and TNF KO mice. Symbols indicate individual mice. One of 3 experiments with similar results is shown (n = 5-8 mice per group). (B) Mice were immunized i.f.p. with 108 SRBCs and anti-SRBC IgG titers were analyzed on day 21. SplX-WT mice were splenectomized 10 days before immunization. LTα KO mice are included as control. Average is indicated. Symbols indicate individual mice. One of 2 independent experiments with similar results is shown (n = 5-8 mice per group); *P < .05, **P < .01, NS, not significant. (C) Administration of TNF blocker inhibits humoral immune response to T-cell–dependent antigen. Mice were immunized i.p. with 108 SRBCs and specific anti-IgG response was measured at day 21. Etanercept (30 mg/kg) was injected twice per week starting from day −6. One of 2 independent experiments with similar results is shown. Each symbol represent individual mouse. Average is indicated; *P < .05.
Inactivation of TNF production by both B and T cells results in impaired humoral response to T-cell–dependent antigen. (A) Mice were immunized i.p. with 108 SRBCs. Sera were collected on day 21, and anti-SRBC response was measured by ELISA. Symbols indicate individual mice. Mean values are indicated. IgG response is reduced both in B-TNF and T-TNF KO mice, however not as severe as in T,B-TNF and TNF KO mice. Symbols indicate individual mice. One of 3 experiments with similar results is shown (n = 5-8 mice per group). (B) Mice were immunized i.f.p. with 108 SRBCs and anti-SRBC IgG titers were analyzed on day 21. SplX-WT mice were splenectomized 10 days before immunization. LTα KO mice are included as control. Average is indicated. Symbols indicate individual mice. One of 2 independent experiments with similar results is shown (n = 5-8 mice per group); *P < .05, **P < .01, NS, not significant. (C) Administration of TNF blocker inhibits humoral immune response to T-cell–dependent antigen. Mice were immunized i.p. with 108 SRBCs and specific anti-IgG response was measured at day 21. Etanercept (30 mg/kg) was injected twice per week starting from day −6. One of 2 independent experiments with similar results is shown. Each symbol represent individual mouse. Average is indicated; *P < .05.
To directly address the role of LN structure in development of immune response to T-cell–dependent antigen, we performed intra footpad (i.f.p.) immunizations, after which the antibody response predominantly originated from the draining LNs rather than from the spleen. Specific anti-SRBC IgG responses were reduced in both T-TNF and B-TNF KO mice, but not as severely as in double T,B-TNF KO and TNF KO mice (Figure 3B). Finally, to exclude a role of the spleen in immune responses initiated after i.f.p. injection, mice were splenectomized and injected i.f.p. with antigen 10 days later. Again, specific IgG titers were significantly reduced in B-TNF KO mice (Figure 3B). These data suggest that correct LN structure is critical for the generation of IgG responses and that TNF signals coming from both B and T cells are required for mounting an efficient immune response.
Finally, we treated WT mice with Etanerpect (p75TNFR-Ig)33 and observed significant disruption of lymphoid microarchitecture (supplemental Figure 9). Specific anti-SRBC IgG levels were also significantly reduced in Etanercept-treated group (Figure 3C). These data demonstrate that pharmacologic TNF inhibition affects the integrity of secondary lymphoid organs and may prevent generation of an efficient humoral immune response to T-cell–dependent antigen.
Soluble, but not membrane-associated, TNF produced by B cells is essential for organization of secondary lymphoid organs
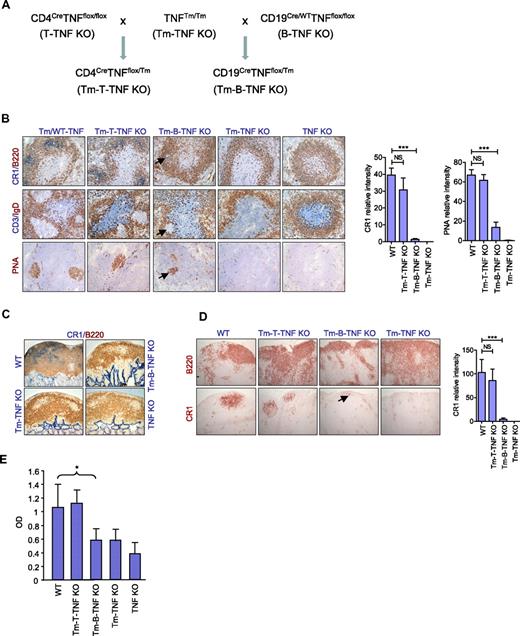
TNF can exist in a membrane-bound form or be secreted as a soluble cytokine that is able to diffuse from the sites of its initial production to lymphoid tissues. It was reported that both soluble and membrane TNF contribute to the organization of secondary lymphoid organs.18 To determine the relative contributions of soluble versus membrane-associated forms of TNF produced by B and T cells in the maintenance of secondary lymphoid organs, we took the advantage of mice with noncleavable TNF18 and generated novel mice expressing only membrane-associated TNF by either B or T cells. In these mice, Cre recombinase deleted only the floxed allele in the desired cell type, but not the noncleavable transmembrane TNF knock-in allele, resulting in elimination of soluble TNF only in either B (Tm-B-TNF KO mice) or T cells (Tm-T-TNF KO mice; Figures 4A and supplemental Figure 10).
Soluble TNF produced by B cells is essential for organization of secondary lymphoid organs and for efficient humoral immune responses. (A) A breeding strategy to generate mice expressing only membrane form of TNF specifically in B or T cells (Tm-B-TNF KO and Tm-T-TNF KO mice). (B-E) Mice were immunized i.p. with 108 SRBCs, and frozen section analyzed on day 8. Representative images from 1 of 2 independent experiments (n = 5 mice per group) are shown. (B) Frozen spleen sections were stained with antibodies anti-B220 (red)/anti-CR1 (blue), PNA (red), anti-CD3(blue)/IgD (red). Arrows indicate residual FDCs and GCs in spleen of Tm-B-TNF KO mice. Original magnification is ×200. Right panels show quantification of FDC and GC areas. Data represent means ± SEM. (C) Frozen sections of PP were stained with anti-CR1 (blue)/anti-B220 (red) antibodies. Original magnification is ×100. (D) Frozen sections of MLNs were stained with anti-B220 and anti-CR1 antibody to visualize B-cell follicles and FDCs, respectively. Arrow indicates residual FDCs staining in MLNs of Tm-B-TNF KO mice. Original magnification is ×100. Right panel shows quantification of FDC areas. Data represent means ± SEM. (E) Soluble TNF from B cells is required for generation of IgG response to T-cell–dependent antigen. Mice were immunized i.f.p. with 108 SRBCs and specific IgG response was measured on day 21. One of 2 independent experiments with similar results is shown. Data represent means ± SD; n = 5 mice per group, *P < .05, **P < .01, ***P < .001.
Soluble TNF produced by B cells is essential for organization of secondary lymphoid organs and for efficient humoral immune responses. (A) A breeding strategy to generate mice expressing only membrane form of TNF specifically in B or T cells (Tm-B-TNF KO and Tm-T-TNF KO mice). (B-E) Mice were immunized i.p. with 108 SRBCs, and frozen section analyzed on day 8. Representative images from 1 of 2 independent experiments (n = 5 mice per group) are shown. (B) Frozen spleen sections were stained with antibodies anti-B220 (red)/anti-CR1 (blue), PNA (red), anti-CD3(blue)/IgD (red). Arrows indicate residual FDCs and GCs in spleen of Tm-B-TNF KO mice. Original magnification is ×200. Right panels show quantification of FDC and GC areas. Data represent means ± SEM. (C) Frozen sections of PP were stained with anti-CR1 (blue)/anti-B220 (red) antibodies. Original magnification is ×100. (D) Frozen sections of MLNs were stained with anti-B220 and anti-CR1 antibody to visualize B-cell follicles and FDCs, respectively. Arrow indicates residual FDCs staining in MLNs of Tm-B-TNF KO mice. Original magnification is ×100. Right panel shows quantification of FDC areas. Data represent means ± SEM. (E) Soluble TNF from B cells is required for generation of IgG response to T-cell–dependent antigen. Mice were immunized i.f.p. with 108 SRBCs and specific IgG response was measured on day 21. One of 2 independent experiments with similar results is shown. Data represent means ± SD; n = 5 mice per group, *P < .05, **P < .01, ***P < .001.
Interestingly, Tm-B-TNF KO mice displayed a phenotype in their secondary lymphoid organs very similar to that observed in B-TNF KO mice (Figure 4B-D). FDCs and organized B-cell follicles were severely reduced in spleen (Figure 4B), PPs (Figure 4C), and LNs (Figure 4D). In addition, staining for MZ-specific markers MOMA-1 and MadCAM-1 in mutant mice indicated that soluble TNF, but not membrane-bound TNF, produced by B cells was essential for the organization of the MZ (supplemental Figure 11). In contrast to Tm-B-TNF KO, Tm-T-TNF KO mice displayed no appreciable defects in the structure of secondary lymphoid organs (Figure 4B-D).
To define the role of soluble TNF produced by B or T cells in the generation of humoral response, we immunized mice with i.p. SRBCs and measured the IgG response on day 21. Specific IgG responses were reduced in Tm-B-TNF KO mice but remained normal in Tm-T-TNF KO mice (Figure 4E), further supporting the essential role of soluble TNF produced by B cells. Overall, our data suggest that it is soluble TNF provided by B cells that plays the essential role in organization of secondary lymphoid organs and in generation of efficient IgG responses.
Discussion
The integrity of intricate organization of the secondary lymphoid organs is essential for generation of an efficient immune response. To define the role of TNF from different cellular sources of hematopoietic origin in vivo for the maintenance of secondary lymphoid organs, we used a genetic approach by inactivating the TNF gene specifically in B or T cells or in both types of lymphocytes. We found that the organization of spleen, LNs, and PPs is maintained by distinct contribution of TNF produced by B and T cells.
One complexity in understanding TNF function in lymphoid organs is because TNF can exert its biologic activity via either membrane-bound or soluble form or both.6,18 Soluble TNF can diffuse from the sites of its initial production to lymphoid tissues, although virtually all splenic cells express TNFR1, which can trap soluble TNF. Thus, it is conceivable that T and B cells may not need to be in direct contact for such TNF-dependent cooperation. Analysis of mice expressing only the membrane form of TNF suggested that both soluble and membrane-bound TNF could play important roles in organization of different secondary lymphoid organs and in host defense.18,34 Our studies using Tm-B-TNF KO mice further demonstrate that soluble TNF produced by B cells is essential for the organization of secondary lymphoid organs and for generation of IgG responses to T-cell–dependent antigens.
The second complexity of interpreting TNF functions in lymphoid organ maintenance is because TNF effects could be mediated by different cell types in different secondary lymphoid organs. Our data suggest that TNF from B cells plays a major role in the maintenance of spleen, LN, and PP microstructure, while TNF from T cells provides a complementary but distinct signal in spleen and MLNs (supplemental Figure 12), but not in PPs. Interestingly, the role of TNF in the maintenance of peripheral LNs appears to be distinct from MLNs and is mainly mediated by TNF signals from B cells (supplemental Figure 13).
Mice with inactivation of LT in B and T cells show relatively normal structure of LNs,15 while inactivation of Tnf gene in T and B cells in T,B-TNF KO mice results in a complete lack of FDCs and organized B-cell follicles. Although the LT and TNF pathways are acting in concert for the maintenance of secondary lymphoid organs, in most of the studied cases the effects of LT ablation are much stronger than those due to TNF inactivation. Such signaling requirements in LNs of adult mice are, to our knowledge, the first example where the role of TNF produced by lymphocytes is more prominent than that of lymphocyte-derived LT.
To define a molecular mechanism for cooperation between TNF producing B and T cells in organization of LNs, we searched for TNF-dependent genes whose expression was affected in LN of conditionally TNF-deficient mice. Our data demonstrate that TNF signals from B and T cells act in concert in the regulation of several genes involved in proper migration of lymphocytes within the LNs, including the chemokines CXCL13 and CCL22. A key unresolved question concerns the nature of specific TNF- and LT-dependent genes driving FDC precursors to develop into mature FDC networks in various lymphoid tissues. Presumably, some of these genes are expressed by stromal cells of lymphoid organs (including FDC precursors themselves) in response to either TNFR1 or LTβR signaling. FDC-specific genes may include those encoding the homeostatic chemokines, such as CXCL13, CCL22, as well as other stromal genes revealed through comparative expression profiling of WT and TNF/LT-deficient mice31,35 or FDC-like cell lines in vitro.36 Interestingly, expression of Cxcl1337 and Cll2238 genes was shown to be regulated by NF-κB. It is possible that optimal expression of these and other critical genes may depend on simultaneous engagement of both LTβR and TNFR1 on the same cell, resulting in simultaneous activation of both classical and alternative NF-κB activation pathways. In line with this assumption, recent data showed that coordination of alternative and classic NF-κB pathways is required for organization of secondary lymphoid organs,39,40 whereas FDC-specific inactivation of the IKKβ-dependent classical NF-κB pathway did not affect the formation of FDC networks.41 Furthermore, inactivation of both LT and TNF signaling pathways in the TNF/LTα/LTβ triple-deficient mice is thought to be responsible for additional defects in the expression of homeostatic chemokines and organization of splenic white pulp,42 compared with single deficient mice.
We found that organization of the PPs in the postnatal period is predominantly dependent on soluble TNF produced by B cells. Our findings are consistent with previous studies suggesting the critical role of B cells in the development of PPs.29,43 Although the exact nature of signals that stimulate TNF production by B cells in PPs remains to be determined, it is possible that local microenvironment, including commensal microflora, can influence production of soluble TNF by B cells. Consistently, a recent study suggested the role of commensal microflora in the development of lymphoid follicles in the gut.44 A possible explanation for the absence of an appreciable role of T cells in organization of PPs is that the sites for the firm adhesion of B and T cells are segregated in PPs and are governed by distinct chemokine gradients. B cells enter HEV in B-cell areas via a CXCL13-dependent mechanism, while T cells can get access only to the interfollicular areas via CCL21 and CCL19 chemokine gradients.45,46
One may envision 2 possible scenarios for the role of TNF in cooperation of T and B cells in homeostasis of secondary lymphoid organs. First, T cells may directly contact B cells in the follicle and provide TNF in a membrane-associated form to promote FDC generation and B-cell functions. Alternatively, soluble TNF released from both B and T cells may act on stromal cells to produce chemokines and other molecules that in turn would promote B cell maturation and ability to support FDC and GC development.
Recent in vivo imaging studies using multiphoton laser-scanning microscopy suggested that follicular reticular cell networks assist the precise migration of B and T cells within parenchyma of LNs.3,47 The ability of antigen-specific B cells to actively migrate within the germinal centers and to contact T cells may facilitate affinity maturation and antibody responses.3 T cell help can be a limiting factor driving selection of higher affinity B-cell clones.3 Our data are consistent with this hypothesis and suggest that TNF may be one of key mediators of such cooperation between antigen-specific T and B cells in generation of efficient immune responses to T-cell–dependent antigens.
Our data using Etanercept demonstrate that TNF blockade results in disturbance of the structure of secondary lymphoid organs and therefore may prevent the generation of an efficient humoral immune response to T-cell–dependent antigen. Our results are consistent with previous studies using various TNF inhibitors on spleen microarchitecture,48-50 but appear at variance with a study, in which mTNFR1-Ig inhibitor was used.51 Apparently, Etanercept had more potent activity compared with mTNFR1-Ig in this model, which may be due to intrinsic properties of these 2 agents.
Collectively, our study has revealed distinct and organ-specific requirements for TNF-mediated signals originating from T and B cells in the development and organization of different secondary lymphoid tissues.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are greatly indebted to J. Takeda, R. Rickert, D. Littman, and C. Wilson for Cre transgenic mouse strains and J. Sedgwick for transmembrane TNF knock-in mice. We thank L. Tessarollo, E. Southon, A. Shakhov, D. Liepinsh, T. Murakami, L. Drutskaya, and T. Stull for their assistance at various stages of this project, D. Schlessinger and Y.-X. Fu for helpful advice and support, and to A. Chervonsky, A. Rudensky, and T. Junt for critical comments on the manuscript. In addition, we thank G. Telegin, A. Hovsepyan, and the personnel of Laboratory Animal Breeding Center, Branch of Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, for expert help with mouse breeding and production.
This project has been funded in part with Molecular and Cell Biology grants from the Russian Academy of Sciences, by grant 09-04-12185 from the Russian Foundation for Basic Research, by Grant of the President of the Russian Federation for support of leading scientific schools (grant 02.120.11.8796), and by Deutsche Forschungsgemeinschaft (SFB633). This work was also in part supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. S.A.N. is International Research Scholar of the Howard Hughes Medical Institute and the recipient of Helmholtz-Humboldt award.
Authorship
Contribution: A.V.T., S.I.G, A.A.K., Y.V.S., E.P.K., Y.P., and C.-Y.C. performed research; and A.V.T., S.I.G, A.A.K., D.V.K., and S.A.N. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dmitry Kuprash, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Vavilov St 32, Moscow 119991, Russia; or Sergei Nedospasov, e-mail: sergei.nedospasov@gmail.com.
References
Author notes
A.V.T., S.I.G., A.A.K., D.V.K, and S.A.N. contributed equally to this study.