Abstract
Microbial infection triggers the endogenous production of immunosuppressive glucocorticoid (GC) hormones and simultaneously activates innate immunity through toll-like receptors (TLRs). How innate immune cells integrate these 2 opposing signals in dictating immunity or tolerance to infection is not known. In this study, we show that human plasmacytoid predendritic cells (pDCs) were highly sensitive to GC-induced apoptosis. Strikingly, they were protected by microbial stimulation through TLR-7 and TLR-9, but not by microbial-independent stimuli, such as interleukin-3, granulocyte macrophage colony-stimulating factor, or CD40-ligand. This protection was dependent on TLR-induced autocrine tumor necrosis factor-α and interferon-α, which collectively increased the expression ratio between antiapoptotic genes (Bcl-2, Bcl-xL, BIRC3, CFLAR) versus proapoptotic genes (Caspase-8, BID, BAD, BAX). In particular, virus-induced Bcl-2 up-regulation was dependent on autocrine interferon-α. Using small interfering RNA technology, we demonstrated that Bcl-2 and CFLAR/c-flip were essential for TLR-induced protection of pDCs from GC-induced caspase-8–mediated apoptosis. Our results demonstrate a novel property of the TLR pathway in regulating the interface between GC and innate immunity and reveal a previously undescribed mechanism of GC resistance.
Introduction
Glucocorticoid (GC) hormones are essential for the homeostasis of complex organisms. They act through the ubiquitously expressed GC receptor (GCR) to regulate a variety of functions, such as electrolyte balance, bone metabolism, sleep cycle, and stress responses.1 Increased GC production is an inherent response to stress, including infection.2 Importantly, GC at different concentrations has different effects. For example, the blood concentration of GC is 10−9M at the steady physiologic state and 10−6M at stress state. Most pharmacologic administration of GC in human patients reaches 10−4M and higher blood concentrations.3-5
GCs play an essential role at the interface between the endocrine and immune systems.1 They have anti-inflammatory and immunoregulatory properties,2,6,7 which are being exploited pharmacologically for the treatment of chronic inflammatory, allergic, and autoimmune diseases.8
A major mechanism by which GCs mediate anti-inflammatory responses is to induce apoptosis of several immune cell types,9 including eosinophils,10 activated T cells,11 thymocytes,3,12 monocytes,4 dendritic cells (DCs),13 and plasmacytoid predendritic cells (pDCs).14 Induction of apoptosis by GCs appear to be more restricted to immune cells, because GCs have antiapoptotic effects on epithelial cells, such as mammary gland, hepatocytes, and ovarian follicular cells.15,16 Another mechanism is that GCs inhibit the production of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, and IL-12, by interfering with the nuclear factor κB (NFκB) pathway.17-21
During infection, microbes can activate immune cells through pattern-recognition receptors, such as toll-like receptors (TLRs).22,23 Simultaneously, infection triggers endogenous GC production as part of the stress response.1 Thus, innate immune cells receive immunosuppressive signals through GCs and immune stimulation through TLR triggering. How they integrate these opposing signals to mount an effective immune response is not known.
In this study, we discovered that TLR ligands induced a complete protection of pDCs from GC-induced apoptosis, allowing pDCs to mount an effective antiviral innate immune response.
Methods
Blood samples and pDC purification
Healthy donor human blood buffy coats were obtained from Etablissement Français du Sang, Paris, Saint-Antoine Crozatier blood bank through an approved convention with the Institut Curie. All donors gave their informed consent for research use of the buffy coats. Experimental procedures with human blood have been approved by the Curie Hospital Ethical Committee for human research and were done in accordance with European Union guidelines and the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-gradient (Amersham). Fresh pDCs were isolated from PBMCs using the negative selection pDC untouched isolation kit (Miltenyi Biotec) followed by fluorescence-activated cell sorting (FACS) using staining (CD11c−, CD4+, CD123+; all from BD Biosciences).
Condition of pDC cultures
Freshly isolated pDCs were cultured in RPMI 1640 (Invitrogen) containing 10% fetal bovine serum (Hyclone). Then cells were exposed 24 hours to several concentration of heat-inactivated influenza virus Flu (PR8 strain; Charles River Laboratories) and GC (Sigma-Aldrich). Rescuing experiments of pDCs were done using several agents such as virus (human simplex virus, HSV; Sendai), Staphylococcus aureus Cowan (SAC), CpG (A, B, and C type; a kind gift from Dr F. Barrat, Dynavax Technologies), lipopolysaccharide (LPS; Sigma-Aldrich), poly(cytidylic-inosinic) acid (poly I:C; Sigma-Aldrich), lipoteichoic acid (LTA; Sigma-Aldrich), peptidoglycan (PGN), recombinant interferon-γ (IFN-γ; R&D Systems), as well as CD40L-transfected L cells. Blocking experiments were done using neutralizing monoclonal antibodies against TNF-α, IFN-α, and type I IFN receptor (IFNAR; R&D Systems and PBL Biomedical Laboratories).
pDC viability and apoptosis assays
After overnight culture, pDCs were collected and viability was assessed using cell count after trypan blue exclusion of dead cells. For apoptosis versus necrosis discrimination, pDCs were stained with annexin V–fluorescein isothiocyanate (FITC)/propidium iodide (PI; Promega) and fluorescence was analyzed by flow cytometry (FACSCalibur; Becton Dickinson).
Type I IFN and TNF-α productions
After overnight pDC culture, supernatants were collected and frozen at −80°C. Type I IFN and TNF-α productions were measured within 3 months of collection using enzyme-linked immunosorbent assay (ELISA; R&D Systems and PBL Biomedical Laboratories).
Quantitative RT-PCR
Total RNA was extracted with RNeasy Micro kit (QIAGEN). A mixture containing random hexamers, oligo(dT)15 (Promega) and SuperScript II reverse transcriptase (Invitrogen) was used for cDNA synthesis. Transcripts were quantified by real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) on an ABI PRISM 7900 sequence detector (Applied Biosystems) with Applied Biosystems predesigned TaqMan Gene Expression Assays and Absolute QPCR ROX mix (Thermo Fisher Scientific). The following probes were used (Applied Biosystems): BCL2 (Hs00608023_m1), BCL2L1 (Hs00236329_m1), CFLAR (Hs00236002_m1), BIRC3 (Hs00154109_m1), CASP8 (Hs01018151_m1), BAX (Hs00751844_s1), BAD (Hs00188930_m1), BID (Hs00609630_m1), HLA-DRA (Hs00219575_m1), TNF (Hs00174128_m1), and IFNA2 (HS 00265051_s1). All cycle thresholds (Cts) were first normalized to the housekeeping gene RPL34 (Hs00241560_m1), and then for each experiment, the sample with the highest value was set to 100%.
siRNA pDC transfection
pDCs were seeded at 106 cells/mL in 96-well plates and incubated at 37°C. Three microliters Hiperfect (QIAGEN) were added to the appropriate siRNA concentration and adjusted at 100 μL with serum-free medium. Then, the solution was gently mixed and incubated at room temperature during 30 minutes. After incubation, the mix was added to cells in culture. Finally, cells were incubated at 37°C for 24 hours with or without influenza virus and GC. Control was performed using scramble small interfering RNA (siRNA) and transfection efficiency was tested by FACS using Alexa 488–tagged scramble siRNA (all from QIAGEN). Apoptosis was determined using annexin V/PI costaining and analyzed by flow cytometry.
siRNA screening
For apoptotic pathway characterization, siRNA duplexes (Sigma Proligo) used were: (5′-AACAUCGCCCUGUGGAUGACU-3′) for Bcl-2, (5′-AAGGAGAUGCAGGUAUUGGUG-3′) for Bcl-xL, (5′-AAGACCAUUCAGAAGAUGCAA-3′) for iap-2, (5′-AAGGUGCCGGAACUGAUCAGA-3′) for BAX, (5′-AACCUCGGGGAUACUGUCUGA-3′) for Caspase-8, and (5′-AACUGCUCUACAGAGUGAGGC-3′) for c-flip.
Results
pDCs are highly sensitive to GC-induced apoptosis and are not rescued by nonmicrobial stimuli
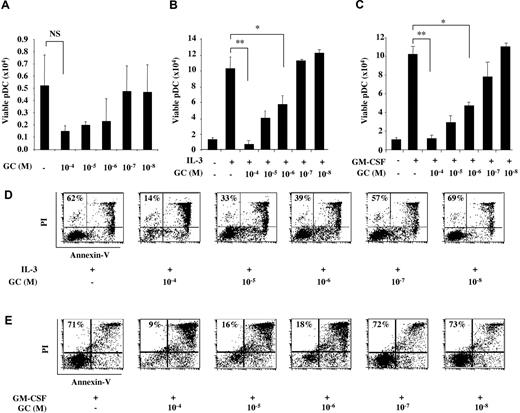
pDCs are sensitive to spontaneous apoptosis when cultured in serum-containing medium in the absence of activating signals.24 Viability of freshly sorted normal blood pDC (> 99% purity) after 24 hours in culture medium was consistently < 30% and was further decreased in a dose-dependent manner in the presence of the natural GC hydrocortisone (Figure 1A), confirming that pDCs are sensitive to GC-induced apoptosis.14 We asked whether IL-3, which promotes pDC survival and activation,25 was able to protect pDCs from GC-induced apoptosis. IL-3 (10 ng/mL) promoted good pDC viability after 24 hours of culture as assessed by trypan blue exclusion of dead cells (Figure 1B). However, addition of GC induced a dose-dependent drop in pDC viability, from more than 80% with pharmacologic concentration (10−4M) to approximately 50% with stress-induced GC concentrations (10−6M; Figure 1B). At homeostatic GC concentration (10−8M), GC did not affect pDC viability (Figure 1B). Increasing IL-3 concentrations up to 1 μg/mL did not rescue pDCs from apoptosis (data not shown). Comparable results were obtained using granulocyte macrophage colony-stimulating factor (GM-CSF), which was efficient in promoting pDC survival26 but did not protect pDCs from GC-induced cell death (Figure 1C). T cell–derived molecules, such as CD40-ligand (CD40-L) and IFN-γ, which were shown to protect pDC from IL-4– and IL-10–induced apoptosis,27 did not prevent GC-induced apoptosis of pDCs (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
pDC are highly sensitive to GC-induced apoptosis and are not rescued by TLR-independent stimuli. Cell viability of freshly isolated pDCs cultured 24 hours in the presence of different GC concentrations (10−4 to 10−8M; A), in association or not with IL-3 (10 ng/mL; B), or with GM-CSF (100 ng/mL; C) were assessed by trypan blue dead cell exclusion. Apoptotic and necrotic cells were quantified by flow cytometry using an annexin V/PI staining. The percentage of cells in each dot plot indicates double-negative viable pDCs (bottom left quadrant) in the presence of various GC concentrations (10−4 to 10−8M) associated to the addition of IL-3 (10 ng/mL; D) and GM-CSF (100 ng/mL; E). Histograms represent the mean ± SD of at least 3 independent experiments. P values were determined using 2-tailed Student t test (NS, not significant; *P < .05; **P < .01).
pDC are highly sensitive to GC-induced apoptosis and are not rescued by TLR-independent stimuli. Cell viability of freshly isolated pDCs cultured 24 hours in the presence of different GC concentrations (10−4 to 10−8M; A), in association or not with IL-3 (10 ng/mL; B), or with GM-CSF (100 ng/mL; C) were assessed by trypan blue dead cell exclusion. Apoptotic and necrotic cells were quantified by flow cytometry using an annexin V/PI staining. The percentage of cells in each dot plot indicates double-negative viable pDCs (bottom left quadrant) in the presence of various GC concentrations (10−4 to 10−8M) associated to the addition of IL-3 (10 ng/mL; D) and GM-CSF (100 ng/mL; E). Histograms represent the mean ± SD of at least 3 independent experiments. P values were determined using 2-tailed Student t test (NS, not significant; *P < .05; **P < .01).
To assess whether GC-induced cell death of pDCs was due to necrosis or apoptosis, we performed annexin V/PI staining of 24-hour–cultured pDCs in the presence of decreasing GC concentrations. IL-3 promoted good pDC viability (62% annexin VnegPIneg viable cells), which dropped to 14% with high-dose GC (10−4M) and 39% with stress-induced concentrations (10−6M; Figure 1D). Dying cells were mostly annexin Vpos, confirming apoptosis. Similar results were obtained with GM-CSF, with annexin VnegPIneg cells dropping from 71% to 9% in the presence of high-dose GC (Figure 1E). Thus, IL-3 and GM-CSF were unable to rescue pDCs from GC-induced apoptosis.
TLR triggering protects pDCs from GC-induced apoptosis
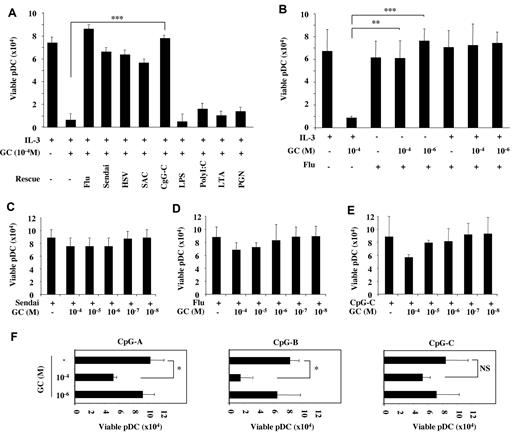
Microbial nucleic acids can directly activate human pDCs through TLR-7 and TLR-9.25 To investigate whether TLR-7/-9 signal could prevent the GC-induced apoptosis, pDCs were cultured with GC in the presence or absence of IL-3 and different TLR-ligands. TLR-7–[influenza virus (Flu) and Sendai virus (Sendai)] and TLR-9–(HSV, SAC, and CpG-C) ligands completely protected pDCs from GC-induced cell death (Figure 2A). TLR-4–(LPS), TLR-3–(poly I:C), and TLR-2–(LTA and PGN) ligands do not activate pDCs25 and did not rescue them from GC-induced cell death (Figure 2A). Of note, pDCs with GC alone were systematically all dead after the overnight culture (Figure 1A), so this negative control was not added in the experiments shown in Figure 2.
TLR triggering protects pDCs from GC-induced apoptosis. (A-B) Viability of pDCs cultured overnight with IL-3 (10 ng/mL) and GC at 10−4M in the presence of TLR-7/-9 ligands (Flu, Sendai virus, HSV, SAC, CpG-C26) and TLR-2/-3/-4 ligands (LPS, poly I:C, LTA, PGN). (C-E) Viability of pDCs cultured overnight with various GC concentrations (10−4 to 10−6M) in the presence of TLR-7/-9 stimulators (Flu, Sendai virus, CpG-C). (F) Viability of pDCs cultured overnight with GC (10−4 and 10−6M) in the presence of different CpG types (CpG-A, -B, and -C). Histograms represent the mean ± SD of at least 3 independent experiments. P values were determined using 2-tailed Student t test (NS, not significant; *P < .05; **P < .01; ***P < .001).
TLR triggering protects pDCs from GC-induced apoptosis. (A-B) Viability of pDCs cultured overnight with IL-3 (10 ng/mL) and GC at 10−4M in the presence of TLR-7/-9 ligands (Flu, Sendai virus, HSV, SAC, CpG-C26) and TLR-2/-3/-4 ligands (LPS, poly I:C, LTA, PGN). (C-E) Viability of pDCs cultured overnight with various GC concentrations (10−4 to 10−6M) in the presence of TLR-7/-9 stimulators (Flu, Sendai virus, CpG-C). (F) Viability of pDCs cultured overnight with GC (10−4 and 10−6M) in the presence of different CpG types (CpG-A, -B, and -C). Histograms represent the mean ± SD of at least 3 independent experiments. P values were determined using 2-tailed Student t test (NS, not significant; *P < .05; **P < .01; ***P < .001).
Next, we asked whether the protection of pDCs required a synergy between TLR triggering and IL-3. We found that Flu was able to maintain pDC viability in the presence of pharmacologic (10−4M) and stress-induced (10−6M) GC levels independently of IL-3 (Figure 2B and supplemental Figure 1B). Similar results were obtained using annexin V/PI staining, confirming the protection from GC-induced apoptosis (see below and Figure 5A). Sendai, Flu, and CpG-C almost completely protected pDCs from apoptosis across different GC concentrations (10−4 to 10−8M; Figure 2C-E). Because different classes of CpG (A, B, and C) differentially induce type I IFN and TNF-α production by pDCs,25,28 we compared their ability to protect pDCs from GC-induced cell death (Figure 2F). Although all 3 types were able to induce protection in the presence of stress-induced GC concentrations (10−6M), CpG-C induced the most efficient protection to high-dose GC (Figure 2F). CpG-A, which mostly triggers a type I IFN response induced partial protection, whereas CpG-B, which mostly promotes TNF-α production and DC differentiation, was not able to rescue pDC viability at pharmacologic GC concentrations (10−4M; Figure 2F). Collectively, these results demonstrate that TLR triggering of pDCs specifically induced complete protection from GC-induced apoptosis, even at pharmacologic GC concentrations.
Autocrine TNF-α and IFN-α play a critical role in the TLR-mediated protection of pDCs from GC-induced apoptosis
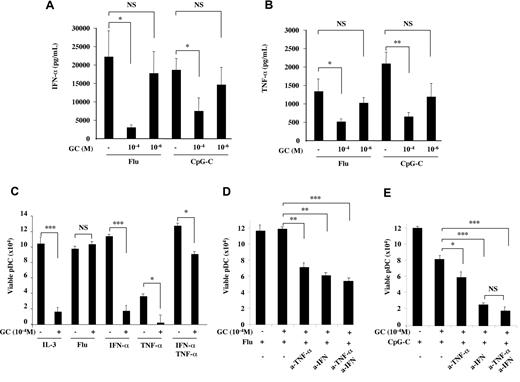
To address the mechanism of TLR-mediated protection of pDCs from GC-induced apoptosis, we first focused on TLR-induced secreted molecules that may play a role in an autocrine manner. After TLR activation, pDCs produce variable levels of type I IFN and TNF-α, which were shown to promote pDC survival and differentiation, respectively.29 Since GC may inhibit cytokine production by pDCs independently of apoptosis, we first assessed the ability of pDCs to produce IFN-α and TNF-α in the presence of GC. IFN-α and TNF-α were decreased by pharmacologic GC concentration (10−4M), despite the conserved pDC viability, but they were still detected at significant levels, around 5 ng/mL for IFN-α and 500 pg/mL for TNF-α (Figure 3A-B). Moreover, concentrations at the cell surface may be higher than those measured in culture supernatant given the autocrine mode of action. Flu- and CpG-induced IFN-α and TNF-α secretion was not significantly affected by stress-induced GC levels (10−6M; Figure 3A-B), suggesting a maintained pDC function in the context of endogenous GC production during infection. Overall, the maintained production of IFN-α and TNF-α at significant levels makes them candidates for an autocrine effect on pDCs.
TLR-induced IFN-α and TNF-α synergize for the protection of pDCs from GC-induced apoptosis. (A-B) Overnight IFN-α and TNF-α production by purified pDCs (105 cells/well) from healthy blood donors was assessed in the presence of Flu or CpG-C in association with GC (10−4 and 10−6M). IFN-α and TNF-α productions were inhibited by pharmacologic, but unaffected by stress-induced, GC levels. (C) Viability of pDCs cultured overnight with GC at 10−4M in the presence of TLR-7 ligand (Flu) or with recombinant cytokines (IFN-α, IL-3, and TNF-α). (D) Viability of pDCs cultured overnight with GC at 10−4M and TLR-7 ligand (Flu) in the presence of IFN-α and TNF-α blocking antibodies. (E) Viability of pDCs cultured overnight with GC at 10−4M and TLR-9 ligand (CpG-C) in the presence of IFN-α and TNF-α blocking antibodies. Histograms represent the mean ± SD of at least 3 independent experiments. P values were determined using 2-tailed Student t test (NS, not significant; *P < .05; **P < .01; ***P < .001).
TLR-induced IFN-α and TNF-α synergize for the protection of pDCs from GC-induced apoptosis. (A-B) Overnight IFN-α and TNF-α production by purified pDCs (105 cells/well) from healthy blood donors was assessed in the presence of Flu or CpG-C in association with GC (10−4 and 10−6M). IFN-α and TNF-α productions were inhibited by pharmacologic, but unaffected by stress-induced, GC levels. (C) Viability of pDCs cultured overnight with GC at 10−4M in the presence of TLR-7 ligand (Flu) or with recombinant cytokines (IFN-α, IL-3, and TNF-α). (D) Viability of pDCs cultured overnight with GC at 10−4M and TLR-7 ligand (Flu) in the presence of IFN-α and TNF-α blocking antibodies. (E) Viability of pDCs cultured overnight with GC at 10−4M and TLR-9 ligand (CpG-C) in the presence of IFN-α and TNF-α blocking antibodies. Histograms represent the mean ± SD of at least 3 independent experiments. P values were determined using 2-tailed Student t test (NS, not significant; *P < .05; **P < .01; ***P < .001).
Next, we addressed whether exogenous IFN-α and TNF-α were sufficient to induce pDC protection from GC-induced apoptosis. No effect was observed on pDC viability when IFN-α and TNF-α were used separately (Figure 3C). However, their combined effect increased pDC survival and induced an almost complete protection from high dose GC (10−4M; Figure 3C). Thus, IFN-α and TNF-α acted synergistically and could recapitulate the effect of TLR stimulation of pDCs. To address the role of endogenously produced IFN-α and TNF-α, we blocked each of these cytokines in pDC cultures in the presence of a TLR ligand together with GC (Figure 3D-E). As we already showed, Flu induced good pDC viability that was not affected by high dose GC (Figure 3D). Further addition of blocking monoclonal antibody (mAb) to IFN-α, TNF-α, or both, restored GC-induced cell death by approximately 50% (Figure 3D), indicating that autocrine IFN-α and TNF-α were involved in pDC protection. In the presence of CpG-C and high dose GC, blocking endogenous TNF-α had only a small, although significant, effect whereas blocking IFN-α decreased pDC viability by more than 50% (Figure 3E). These may explain why CpG-C, which triggers both IFN-α and TNF-α production, promotes complete protection from GC-induced apoptosis, as opposed to CpG-A and B, which mostly induce IFN-α and TNF-α, respectively, and are not sufficient to protect pDC at high GC concentrations (Figure 2F).
TLR-dependent and -independent stimuli differentially modulate the balance between pro- and antiapoptotic molecules in pDCs
To characterize the molecular pathways underlying the TLR-dependent protection of pDCs from GC-induced apoptosis and the contribution of autocrine IFN-α and TNF-α in regulating such pathways, we measured the expression of a panel of pro- and antiapoptotic molecules by quantitative PCR (Figure 4). Some of them were involved in the proapoptotic effect of GC on other cell types.15 Most of the data were obtained after 6 hours of pDC culture with IL-3 (TLR-independent) or Flu (TLR-dependent), and compared with ex vivo and medium-cultured pDCs. At this time point, we verified that both IL-3 and Flu induced an up-regulation of HLA-DR, which was more pronounced with Flu (supplemental Figure 2A). Only Flu induced significant IFN-α mRNA expression (supplemental Figure 2B), whereas TNF-α mRNA was induced by IL-3 and Flu (supplemental Figure 2C).
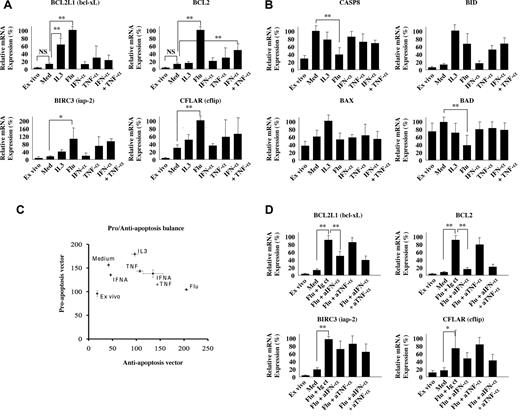
TLR triggering specifically induces multiple antiapoptotic molecules in pDCs. Quantitative RT-PCR of antiapoptotic genes, BCL2L1, BCL2, BIRC3, and CFLAR (A), and proapoptotic genes, CASP-8, BID, BAX, and BAD (B). For each donor the condition with the highest relative expression was set to 100%. Experiments were performed on freshly purified pDCs (ex vivo) or after 6 hours of culture in the presence of medium alone (Med), IL3 (IL-3), Flu, IFN-α, TNF-α, and IFN-α plus TNF-α (A-B). Planar representation of proapoptotic versus antiapoptotic balance in stimulated pDCs (C). To compress the data, one vector x = (BCL2L1 = Bcl-XL, Bcl2, BIRC3 = iap2, CFLAR = c-flip) of antiapoptotic markers, and another vector y = (CASP8 = caspase-8, BID, BAX, BAD) of proapoptotic markers were defined. Then, Euclidean length was computed using the percentage values donor by donor. For each culture condition, the corresponding point is the mean length of 3 independent donors. The error bars correspond to the SDs. Quantitative RT-PCR of antiapoptotic genes, BCL2L1, BCL2, BIRC3, and CFLAR, of Flu-stimulated pDCs in the presence of blocking IFN-α, TNF-α, or both (D). The mean and SD of 3 independent donors are represented. Differential expression between conditions was assessed by Student t test (NS, not significant; *P < .05; **P < .01).
TLR triggering specifically induces multiple antiapoptotic molecules in pDCs. Quantitative RT-PCR of antiapoptotic genes, BCL2L1, BCL2, BIRC3, and CFLAR (A), and proapoptotic genes, CASP-8, BID, BAX, and BAD (B). For each donor the condition with the highest relative expression was set to 100%. Experiments were performed on freshly purified pDCs (ex vivo) or after 6 hours of culture in the presence of medium alone (Med), IL3 (IL-3), Flu, IFN-α, TNF-α, and IFN-α plus TNF-α (A-B). Planar representation of proapoptotic versus antiapoptotic balance in stimulated pDCs (C). To compress the data, one vector x = (BCL2L1 = Bcl-XL, Bcl2, BIRC3 = iap2, CFLAR = c-flip) of antiapoptotic markers, and another vector y = (CASP8 = caspase-8, BID, BAX, BAD) of proapoptotic markers were defined. Then, Euclidean length was computed using the percentage values donor by donor. For each culture condition, the corresponding point is the mean length of 3 independent donors. The error bars correspond to the SDs. Quantitative RT-PCR of antiapoptotic genes, BCL2L1, BCL2, BIRC3, and CFLAR, of Flu-stimulated pDCs in the presence of blocking IFN-α, TNF-α, or both (D). The mean and SD of 3 independent donors are represented. Differential expression between conditions was assessed by Student t test (NS, not significant; *P < .05; **P < .01).
Next, we measured the expression of antiapoptotic molecules, which may counterbalance the proapoptotic effects of GC. We found that Flu induced the highest levels of Bcl-2 and Bcl-xL expression, whereas IL-3 did not significantly up-regulate Bcl-2 (Figure 4A). Flu also significantly up-regulated BIRC3 and CFLAR/c-flip to higher levels than IL-3 (Figure 4A). Exogenous IFN-α and TNF-α partially recapitulated the effects of Flu, in particular on Bcl-2 up-regulation, with a more pronounced effect of TNF-α compared with IFN-α at this time-point. Kinetic studies revealed that differences in antiapoptotic gene expression were maximal at 3 and 6 hours and were still observed at 12 hours for Bcl-2 and CFLAR/c-flip in response to Flu, whereas BIRC3 and Bcl-xL expression decreased from 3 to 12 hours (supplemental Figure 2D). Interestingly, we also observed a late effect of exogenous IFN-α at 12 hours on up-regulating the antiapoptotic genes Bcl-2, BIRC3, Bcl-xL, and CFLAR/c-flip (supplemental Figure 3A), which was not observed at 6 hours (Figure 4A), illustrating the importance of kinetic measurements to accurately assess the regulation of gene expression.
By contrast, expression of the proapoptotic molecules Caspase-8 and BAD was decreased in the presence of Flu (Figure 4B). BAX expression was not significantly affected by IL-3 and Flu. BID was up-regulated by Flu similar to IL-3 (Figure 4B). IFN-α and TNF-α only marginally modulated these pathways (Figure 4B). On kinetic measurements, Flu-induced down-regulation of Caspase-8 was even more striking at 3 hours of culture (supplemental Figure 2E), whereas TNF-α seemed to promote the later (12 hours) down-regulation of Caspase-8, BAX, and BAD (supplemental Figure 3B).
Cell death and survival depend on the balance between pro- and antiapoptotic pathways, rather than individual molecules.30 To evaluate this balance in human pDCs, we projected the expression values of proapoptotic (Caspase-8, BID, BAD, BAX) and antiapoptotic (Bcl-2, Bcl-xL, BIRC3, CFLAR/c-flip) molecules along 2 vectors, the proapoptosis (y-axis) and the antiapoptosis (x-axis), which generated a composite score (Figure 4C). Each dot of the graph represents the normalized mean score, and the bars indicate standard deviation for each culture condition. In medium alone, we observed a strong and exclusive increase along the proapoptosis vector (y-axis; Figure 4C), in accordance with the spontaneous apoptosis of pDC in the absence of survival stimuli.25 Addition of IL-3 induced a shift along the antiapoptosis vector (x-axis; Figure 4C), which may be sufficient to rescue pDCs from spontaneous apoptosis. Importantly, Flu induced the strongest shift along the antiapoptosis vector compared with medium (x-axis), accompanied by a small decrease in the proapoptotic score (Figure 4C). As was observed for individual antiapoptotic genes, exogenous IFN-α and TNF-α partially recapitulated the effects of Flu, with a shift along the antiapoptosis vector and a predominant contribution of TNF-α compared with IFN-α at this time-point (Figure 4C). The Flu-induced antiapoptotic shift was observed at all time-points tested on kinetic measurements (3, 6, and 12 hours; supplemental Figure 2F).
To address whether autocrine IFN-α and TNF-α were required for the Flu-induced up-regulation of antiapoptotic genes, we measured their expression in the absence or presence of blocking antibodies to IFN-α, TNF-α, or both (Figure 4D). These studies revealed a critical role for TLR-induced IFN-α, which was completely required for Flu-induced Bcl-2 induction (Figure 4D). Autocrine IFN-α also contributed to bcl-xL and CFLAR/c-flip expression, although to a lesser extent (Figure 4D). Blocking TNF-α had minor effects on antiapoptotic gene expression induced by Flu (Figure 4D), suggesting that other pathways downstream of TLR may compensate. Overall, our data demonstrated that TLR stimulation of pDCs increased the expression ratio between antiapoptotic versus proapoptotic genes in a manner dependent on autocrine IFN-α and TNF-α.
Bcl-2 and CFLAR/c-flip are essential to protect pDCs from GC-induced apoptosis
To identify the molecular pathways underlying the protection of pDCs from GC-induced apoptosis, we used siRNA-mediated inhibition of TLR-induced antiapoptotic molecules. Performing siRNA-mediated inhibition on primary cells presents many difficulties, and we had to optimize our methodology to (1) preserve pDC viability, (2) reach a sufficient transfection rate, and (3) minimize pDC activation, since siRNA can bind to TLR-7.31 After comparing a variety of transfection methods, we found that electroporation induced a high rate of pDC death (data not shown). Lipofection gave the best results, with no significant modification of pDC viability (supplemental Figure 4A) and approximately 80% transfection efficacy at an siRNA concentration of 160 nM and higher, as assessed using a fluorescent Alexa-tagged siRNA (supplemental Figure 4B). Control siRNA induced a slight up-regulation in CD40 expression indicating slight pDC activation (supplemental Figure 4C).
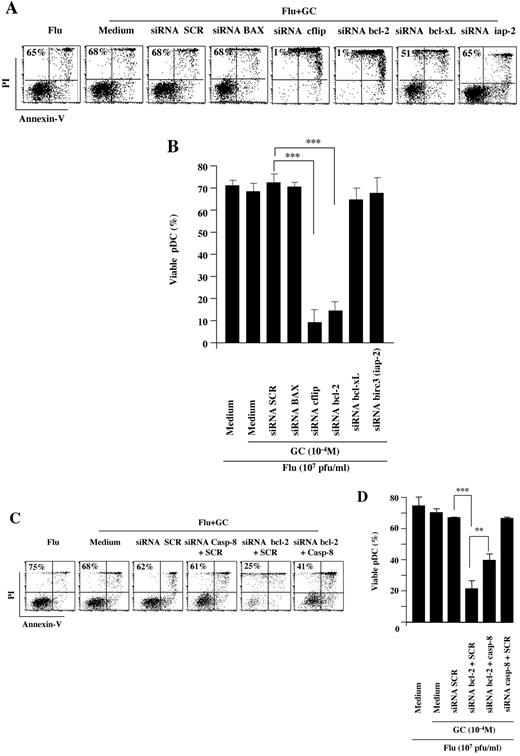
We designed several siRNA sequences interfering with the transcription of antiapoptotic molecules that were found up-regulated by Flu (Figure 4A). We assessed the effect of these siRNA on GC-induced apoptosis of pDC (Figure 5A). Flu activation of pDCs induced approximately 65% viability, as assessed by annexin V/PI staining, and this was not modified by addition of high-dose GC (10−4M; Figure 5A-B). We found that inhibition of the proapoptotic molecule BAX did not increase pDC apoptosis, similar to a scramble siRNA (Figure 5A-B). Inhibition of Bcl-xL and birc-3/iap-2 did not significantly modify pDC survival, with 51% and 65% viable pDC, respectively (Figure 5A-B). In contrast, inhibition of Bcl-2 and c-flip induced a dramatic decrease in pDC viability down to 1%, with an increase in annexin Vpos cells (Figure 5A-B). We further verified that siRNA-mediated inhibition of Bcl-2 transcription in Flu-activated pDCs was accompanied by a decrease in Bcl-2 protein levels by flow cytometry, confirming specificity (supplemental Figure 4D). This demonstrated that Bcl-2 and c-flip were responsible for the protection of pDCs from GC-induced apoptosis.
Bcl-2 and CFLAR/c-flip are essential to protect pDCs from GC-induced apoptosis. (A-B) pDCs stimulated overnight by Flu were exposed to GC in the presence of several siRNA targeting proapoptotic (BAX) or antiapoptotic (c-flip, Bcl-2, iap-2, Bcl-xL) molecules or scramble control siRNA (SCR). (C-D) pDCs stimulated overnight by Flu with GC in the presence of several siRNA targeting proapoptotic (caspase-8) or antiapoptotic (Bcl-2) proteins or SCR. Dot plots show apoptosis assessed using annexin V/PI staining of representative experiment (A,C). Histograms show the results obtained with 7 independent experiments (B,D). In all panels, percentage indicates the live cells in each condition. P values were determined using 2-tailed Student t test (***P < .001).
Bcl-2 and CFLAR/c-flip are essential to protect pDCs from GC-induced apoptosis. (A-B) pDCs stimulated overnight by Flu were exposed to GC in the presence of several siRNA targeting proapoptotic (BAX) or antiapoptotic (c-flip, Bcl-2, iap-2, Bcl-xL) molecules or scramble control siRNA (SCR). (C-D) pDCs stimulated overnight by Flu with GC in the presence of several siRNA targeting proapoptotic (caspase-8) or antiapoptotic (Bcl-2) proteins or SCR. Dot plots show apoptosis assessed using annexin V/PI staining of representative experiment (A,C). Histograms show the results obtained with 7 independent experiments (B,D). In all panels, percentage indicates the live cells in each condition. P values were determined using 2-tailed Student t test (***P < .001).
Bcl-2 can exert its antiapoptotic function through inhibition of caspase-8, but also through caspase-8–independent mechanisms.30 To discriminate between these 2 pathways, we inhibited caspase-8 using a specific siRNA (Figure 5C-D). As described previously, Bcl-2 inhibition using a specific siRNA restored GC-induced apoptosis in the presence of Flu (Figure 5C-D). Further inhibition of caspase-8 significantly increased pDC survival, from approximately 20% to 40%, indicating that bcl-2–mediated protection was caspase-8–dependent (Figure 5C-D).
Discussion
In this study, we demonstrate that TLR activation of human pDCs protected from GC-induced apoptosis, which may represent an important mechanism to preserve innate immune function during infection. This underlines the importance of studying the integration of diverse stimuli by innate immune cells to better understand immune modulation during inflammation. To this end, experimental assessment of this integration is a critical initial step because in most cases, the outcome of signal integration cannot be predicted by the effect of individual stimuli.
During inflammation, cytokines represent important molecules of the microenvironment that mediate immune activation. IL-3 and GM-CSF are the 2 main cytokines able to activate human pDCs in a TLR-independent manner. They are mostly produced by Th2 cells, monocytes/macrophages, basophils, and mast cells32,33 and may be involved in pDC activation during nonmicrobial inflammation, such as allergy or cancer. Our data show that in such conditions pDCs remain very sensitive to GC-induced apoptosis, indicating that the proapoptotic effect of GC is dominant over antiapoptotic signals delivered by inflammatory cytokines. Induction of pDC apoptosis may participate in the therapeutic effects of GC in allergy34,35 but may also be involved in immune contraction induced by endogenous GC, since we observed this effect even at stress-induced concentrations of the natural GC hydrocortisone (10−6M). This is different from monocytes, which die by apoptosis only when exposed to pharmacologic doses of the synthetic GC dexamethasone.4
Microbial inflammation constitutes a complex microenvironment where pro- and anti-inflammatory factors are simultaneously present. The outcome of the infection critically depends on how immune cells will integrate these opposing signals. GC hormones are important endogenous anti-inflammatory molecules. However, most studies on immune cells addressed their role as a single agent,17-21 which does not reflect the complexity of a physiopathologic situation. Indeed, proinflammatory molecules may confer GC resistance to immune cells and hamper the therapeutic anti-inflammatory effects of GC.36 In particular, macrophage migration inhibitory factor (MIF) was able to counter-regulate some immunosuppressive effects of GC, such as the inhibition of proinflammatory cytokine production by LPS-stimulated monocytes.37 The GCR-β isoform, which can be induced by inflammatory cytokines, was also shown to act as an endogenous inhibitor of GC action.38,39 In our study, we demonstrated that direct TLR stimulation of pDCs protected them from GC-induced apoptosis. Thus, microbial-induced TLR activation constitutes a novel mechanism of resistance to endogenous and exogenous GC during infection.
TLR triggering of pDCs and DCs mostly induces cytokine production together with a maturation program leading to immunity through efficient T-cell stimulation.22,23,40 Our data reveal a novel property of the TLR pathway in counteracting the immunosuppressive effects of GC by inducing high levels of the antiapoptotic molecules Bcl-2, Bcl-xL, and CFLAR/c-flip. Thus, TLR-induced antiapoptotic effects on pDCs are dominant over proapoptotic GC, which may promote an efficient antimicrobial immunity during the acute phase of an infection. Conversely, it was shown that the HIV-1 protein vpr could act as a coactivator of the human GC receptor and may increase tissue sensitivity to GC.41 Thus, different microbial stimuli engaging different molecular pathways may act as coactivator or repressor of GC action and differentially promote immunity or tolerance to infection.
In summary, our study sheds new light on how immune cells integrate anti-inflammatory and proinflammatory signals during infection. The dominance of TLR activation over immunosuppressive GC may be critical to promote an efficient antimicrobial immune response. Therapeutic manipulation of the TLR pathway using specific agonists may offer new means of protecting immune cells to avoid deleterious GC-induced immunosuppression. Conversely, TLR antagonists may be used to restore GC sensitivity in GC-resistant inflammatory and autoimmune diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Zofia Maciorowski and Annick Viguier for cell sorting, and Dr J. A. Ribeil (Department of Biotherapy, Hospital Necker-Enfants Malades) for providing us with blood samples.
R.Z. was supported by the European Community Sixth Framework Program (grant EXT 014162 to V.S.) and by a fellowship from the Fondation pour la Recherche Médicale (FRM). C.G. was supported by a fellowship from Association pour la Recherche contre le Cancer (ARC). A.C. was supported by a grant from Agence National pour la Recherche (ANR). Y.L. is the recipient of a grant from Association pour la Recherche contre le Cancer.
Authorship
Contribution: Y.L. and V.S. designed the study, carried out experiments, collected, analyzed and interpreted data, and assisted in writing the manuscript; O.H. and Y.-J.L. assisted in writing the manuscript; R.Z. and C.G. carried out experiments, collected data, analyzed and interpreted data, and assisted in writing the manuscript; and R.H.-S., F.R., and A.C. carried out experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vassili Soumelis, Institut Curie, Laboratoire d'Immunologie Clinique, 26 rue d'Ulm, 75005 Paris, France; e-mail: vassili.soumelis@curie.net.
References
Author notes
Y.L., R.Z., and C.G. contributed equally to this study.