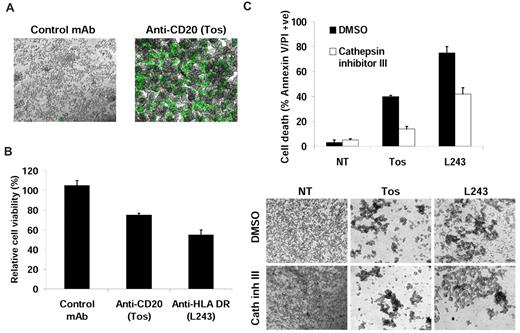
Golay et al raise several important points regarding the possible pitfalls in assessing cell death by flow cytometry. In particular, they refer to experiments on anti-CD20 and HLA-DR monoclonal antibodies (mAbs) reported by our groups in the past year.1,2 We fully concur that more than one technique should be performed to validate any cell death phenomenon and that flow cytometry alone is insufficient. For exactly these reasons we performed real-time and snapshot light microscopic analysis, electron microscopic, and growth inhibition assays to demonstrate by 4 different techniques that type II anti-CD20 and HLA-DR mAbs elicit a novel form of lysosomal-dependent death related to homotypic adhesion (HA) and that type I anti-CD20 mAbs do not. The central argument presented by Golay et al is that cells undergoing HA become sensitive to pipetting and flow cytometric analysis, although it is unclear as to why this should be the case. Nevertheless, we reported in situ experiments with SYTOX green (Invitrogen) that excluded flow cytometry and pipetting. Importantly in these experiments, where there was no mechanical disruption, HA was accompanied by increased SYTOX green positivity, indicative of cell death (Figure 1A and Ivanov et al1 ). We also reported a clear and substantial decrease in viable cell proliferation in the presence of tositumomab or HLA-DR mAb, using XTT assays (Figure 1B and Ivanov et al1 ). This assay again excludes the mechanical issues of pipetting and flow cytometry detailed by Golay et al. Both the XTT reagent used by us and the alamarBlue used by Golay et al operate through a common mechanism (reduction by metabolically active cells to give a color change). Therefore, the differences observed between our groups are hard to reconcile, but may potentially be explained by differences in cell density or dye incubation time used. Furthermore, we have previously confirmed that the loss of cell viability observed translated into a loss of clonogenic survival.3
Monoclonal antibody (mAb)–induced cell death detected by flow cytometry is not an artefact of cell aggregation. Alternative assays used to detect mAb-induced cell death. (A) RAJI cells were treated with mAb (10 μg/mL) for 4 hours and labeled in situ with SYTOX green. Cell death was then analyzed by fluorescence microscopy (original magnification: ×4), representative of 2 independent experiments. (B) Cells were treated with mAb (10 μg/mL) for 24 hours and cell viability determined by the colorimetric XTT assay. Cells were incubated for 4 hours at 37°C with XTT reagent, and absorbance was normalized to untreated cells. Mean ± SEM of 3 independent experiments are shown. (C) Cells were pretreated with Cathepsin inhibitor III (100μM) for 30 minutes before treatment with mAb and after 4 hours cell death and cell aggregation were analyzed by flow cytometry (top panel) and microscopy (bottom panel), respectively (original magnification: ×4). Mean ± SEM of 3 independent experiments are shown. Cathepsin inhibitor III potently attenuated cell death induced by both tositumomab (anti-CD20) and L243 (anti-HLA DR) but had no effect on cell aggregation. NT indicates nontreated; Tos, Tositumomab/B1; and control mAb, anti-CD3 (OKT3).
Monoclonal antibody (mAb)–induced cell death detected by flow cytometry is not an artefact of cell aggregation. Alternative assays used to detect mAb-induced cell death. (A) RAJI cells were treated with mAb (10 μg/mL) for 4 hours and labeled in situ with SYTOX green. Cell death was then analyzed by fluorescence microscopy (original magnification: ×4), representative of 2 independent experiments. (B) Cells were treated with mAb (10 μg/mL) for 24 hours and cell viability determined by the colorimetric XTT assay. Cells were incubated for 4 hours at 37°C with XTT reagent, and absorbance was normalized to untreated cells. Mean ± SEM of 3 independent experiments are shown. (C) Cells were pretreated with Cathepsin inhibitor III (100μM) for 30 minutes before treatment with mAb and after 4 hours cell death and cell aggregation were analyzed by flow cytometry (top panel) and microscopy (bottom panel), respectively (original magnification: ×4). Mean ± SEM of 3 independent experiments are shown. Cathepsin inhibitor III potently attenuated cell death induced by both tositumomab (anti-CD20) and L243 (anti-HLA DR) but had no effect on cell aggregation. NT indicates nontreated; Tos, Tositumomab/B1; and control mAb, anti-CD3 (OKT3).
Although HA correlates with cell death, the relationship is not direct for all mAbs. For example, the pan-HLA mAb A9-1 caused high levels of aggregation yet elicited relatively low levels of cell death compared with anti-HLA DR and type II anti-CD20 mAbs.1 This lack of a direct relationship implies that mechanisms other than the simple mechanical explanation proposed link HA and cell death.
Finally, we have demonstrated that mAb-induced cell death is dependent on lysosomal permeabilization and release of cathepsins, using a specific cathepsin inhibitor that potently attenuated cell death measured by flow cytometry but had no effect on HA (Figure 1C). Therefore, the hypothesis that cell death detected by flow cytometry is an artefact of HA cannot explain the prominent inhibition of cell death by the cathepsin inhibitor.
In conclusion, the novel mechanism of antibody-induced death that we have described is definitively not an artefact of pipetting and flow cytometric analysis, but represents a distinctive form of cell death. All cell death phenomena should be validated by different techniques, and if possible, by agents that can modulate cell death without affecting HA, as we have previously demonstrated.1

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal