Abstract
We evaluated the efficacy and safety of romiplostim, a thrombopoietin mimetic, in patients with low- or intermediate-risk myelodysplastic syndromes (MDS) receiving azacitidine therapy. Forty patients with low- or intermediate-risk MDS were stratified by baseline platelet counts (< 50 vs ≥ 50 × 109/L) and randomized to romiplostim 500 μg or 750 μg or placebo subcutaneously once weekly during 4 cycles of azacitidine. The primary endpoint was the incidence of clinically significant thrombocytopenic events, defined by grade 3 or 4 thrombocytopenia starting on day 15 of the first cycle or platelet transfusion at any time during the 4-cycle treatment period. No formal hypothesis testing was planned. The incidence of clinically significant thrombocytopenic events in patients receiving romiplostim 500 μg, romiplostim 750 μg, or placebo was 62%, 71%, and 85%, respectively. The incidence of platelet transfusions was 46%, 36%, and 69%, respectively. These differences were not statistically significant with the small numbers in each group. Romiplostim 750 μg significantly raised median platelet counts during cycle 3 on day 1 (P = .0373) and at the nadir (P = .0035) compared with placebo. Grade 3 rash and arthralgia each were reported in 1 romiplostim-treated patient (4%). This study suggests romiplostim may provide clinical benefits in MDS patients during azacitidine therapy. This study was registered at www.clinicaltrials.gov as #NCT00321711.
Introduction
Myelodysplastic syndromes (MDSs) are a heterogeneous group of stem cell disorders characterized by ineffective hematopoiesis, peripheral cytopenias, progressive bone marrow failure, and increased risk for transformation into acute myeloid leukemia (AML).1-3 Cytogenetic abnormalities are found in approximately one-half of newly diagnosed cases.4,5 Prognosis is estimated by the International Prognostic Scoring System (IPSS) with 4 risk groups (low, intermediate-1, intermediate-2, and high) for disease progression to AML,6 and median survival ranges from 5.7 years in low-risk MDS to 0.4 years in high-risk MDS. A recent survey of physicians in the United States revealed that approximately 60% to 70% of newly diagnosed patients have low- or intermediate-1 risk MDS.7
Thrombocytopenia is a significant clinical problem in MDS and an independent adverse risk factor for survival.8 Thrombocytopenia is found in approximately 35% to 65% of patients, and hemorrhagic complications lead to death in 14% to 24% of cases.9,10 Therapeutic options for MDS are limited, particularly for older patients with comorbid conditions or organ dysfunction, and existing therapies may cause or exacerbate thrombocytopenia. Azacitidine, a DNA methyltransferase inhibitor, is approved by the Food and Drug Administration for treating MDS, producing objective complete response rates of 15%.11,12 Azacitidine causes dose-dependent myelosuppression, producing severe thrombocytopenia in approximately 60% of patients and leading to treatment delays or dose reductions in approximately 80%.11,13 Platelet transfusions are the only treatment option for thrombocytopenia in MDS13 but may cause febrile and allergic reactions and, rarely, transmission of viral infections, graft-versus-host disease, and anaphylactic reactions.14-16
Romiplostim, a novel peptibody protein, increases platelet production by binding to the thrombopoietin receptor and activating downstream signaling.17,18 Romiplostim is approved for treating thrombocytopenia in patients with chronic immune thrombocytopenia; it is not indicated for use in patients with MDS.19 In a recent study, single-agent romiplostim at subcutaneous doses of 300 to 1500 μg weekly produced platelet responses in 40% to 50% of patients with lower-risk MDS and severe thrombocytopenia.20 This study was designed to evaluate the efficacy and safety of romiplostim in reducing the incidence of clinically significant thrombocytopenia in patients with MDS receiving azacitidine.
Methods
Patients
Patients 18 years of age or older with MDS diagnosed by bone marrow biopsy based on the World Health Organization (WHO) classification were eligible if they had IPSS low, intermediate-1, or intermediate-2 risk, and were to be treated with azacitidine for at least 4 cycles. IPSS scores were determined by the central laboratory. Eligibility required Eastern Cooperative Oncology Group performance status 0 to 2, adequate liver function, and serum creatinine ≤ 2 mg/dL. All patients provided written informed consent. Patients were excluded if they had previously received more than 3 cycles of azacitidine or any hypomethylating agent within 30 days, or had a history of leukemia, aplastic anemia, or bone marrow transplantation, prior malignancy unless treated with curative intent and without disease evidence for ≥ 3 years, active infection, uncontrolled cardiovascular disease, recent myocardial infarction or arterial thrombosis, or history of venous thrombosis or current use of anticoagulation therapy. Patients who received interleukin-11 or any experimental drug or device within 4 weeks of screening or previously received another thrombopoietic growth factor were ineligible.
Study design
This study was conducted in accordance with the principles of the Declaration of Helsinki and its amendments and in compliance with guidelines of Good Clinical Practice and all local regulations. The protocol and informed consent form were approved by an independent ethics committee or institutional review board at each study site. This study is registered at www.clinicaltrials.gov as #NCT00321711.
This phase 2, multicenter, randomized, placebo-controlled study consisted of a treatment phase followed by a 6-month extension phase. Only the prespecified analysis of the first 4 treatment cycles is detailed herein. Azacitidine was administered to all patients according to its approved regimen (75 mg/m2 subcutaneously daily for the first 7 days of each 28-day cycle). Eligible patients were stratified by baseline platelet counts (≥ 50 vs < 50 × 109/L) and then randomized 1:1:1 to romiplostim 500 μg, romiplostim 750 μg, or placebo subcutaneously weekly starting on day 1 of the first azacitidine cycle. Study drug was withheld if platelet counts exceeded 450 × 109/L, and resumed at the next scheduled dosing day after platelet counts had decreased < 200 × 109/L. In accordance with its package insert, azacitidine was delayed in 1-week increments for adverse events. If platelet or neutrophil counts had not recovered by day 42 of a cycle, a 50% lower dose was administered starting on day 42.11 When azacitidine was delayed, treatment with romiplostim or placebo was continued. After completing the treatment phase, the study was unblinded, and patients were eligible to receive weekly treatment with romiplostim in the extension phase.
Concomitant treatments deemed necessary to provide adequate supportive care, including growth factors, were allowed at the investigator's discretion, except for myelosuppressive chemotherapy, histone deacetylase inhibitors, immunomodulating agents, or other medications known or suspected of affecting platelet production. Rescue medication was administered only when patients were considered at immediate risk. Platelet transfusions were administered according to standard practice at each institution, with the American Society of Clinical Oncology guidelines provided to investigators as suggested criteria.21 A unit of platelets was defined as a single unit of platelet-rich plasma or buffy-coat concentrate or 1 apheresis (single-dose) concentrate.
Assessments
Complete blood counts with differential and blood chemistry were monitored weekly as were adverse events and concomitant medication use. Vital signs were measured biweekly, and physical examinations were conducted at the start of each treatment cycle, at the end of treatment, and at study end 3 weeks thereafter. A bone marrow biopsy and aspirate with cytogenetics and an IPSS assessment were performed at the end of treatment visit, 4 weeks after the last dose of study drug. Bone marrow morphology and histology from samples taken before treatment and at end of study were reviewed by a central laboratory. Diagnosis of AML was established using WHO criteria of ≥ 20% blasts in either bone marrow or peripheral blood that persisted for ≥ 4 weeks after drug discontinuation. Patients who did not fulfill WHO criteria but subsequently received treatment for AML were considered to have progressed to AML.
End points
The primary efficacy endpoint was the incidence of clinically significant thrombocytopenic events (CSTEs), defined as Common Terminology Criteria for Adverse Events, Version 3, grade 3 or 4 thrombocytopenia (platelet count < 50 × 109/L), starting on day 15 of the first cycle or by a platelet transfusion at any time during the treatment period. Secondary efficacy endpoints measured during the treatment period included incidence of platelet transfusions, frequency and number of units transfused, incidence of azacitidine dose reduction, or delay resulting from thrombocytopenia, and response rate at the end of azacitidine treatment. MDS treatment responses to azacitidine were defined according to the International Working Group 2006 remission criteria.22 Safety was determined by the incidence of adverse events. Exploratory analyses were conducted to assess the incidence of severe thrombocytopenia by treatment cycle, the platelet nadir during each cycle, and the platelet response (hematologic improvement in platelets).22 Platelet responses were determined 1 week after the last dose of romiplostim in all patients who had a baseline platelet count < 100 × 109/L.
Statistical analyses
Estimated incidence rates of CSTE (65% placebo, 15% romiplostim) suggested an initial sample size of 12 patients per treatment arm, given a 2-sided 95% confidence interval for the difference between placebo and romiplostim extending 0.337 from the observed difference in proportions. The incidences of CSTE, platelet transfusions, and azacitidine dose reduction and delays resulting from thrombocytopenia were summarized by treatment group, and the 95% exact binomial confidence interval for the incidence was calculated for each group and for the difference between groups. Adverse events were summarized according to the Medical Dictionary for Regulatory Affairs, and severity was graded according to Common Terminology Criteria for Adverse Events, Version 3.0. Because of the small sample sizes, all comparisons between treatment groups were descriptive only. No formal hypothesis testing was planned.
Results
Patient disposition and demographics
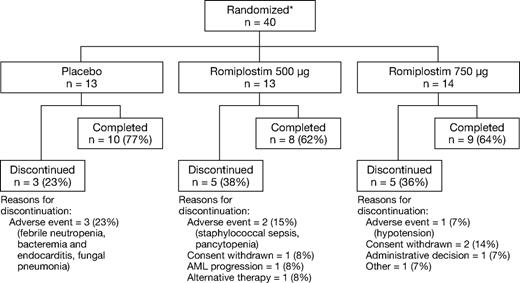
Twenty-seven of 40 randomized patients (68%) completed 4 cycles of azacitidine and study drug (Figure 1). Six patients discontinued because of adverse events, none of which was considered related to romiplostim or placebo. The study cohort had a median age of 71 years (Table 1). Twenty-four patients (60%) had severe thrombocytopenia at baseline, and 30 patients (75%) had previously received platelet transfusions. Most patients had IPSS intermediate-1 (58%) or intermediate-2 (35%) risk. Given the small cohort, there were some minor imbalances in disease characteristics. The placebo group had a higher proportion with IPSS intermediate-2 risk than the romiplostim groups. The distribution of MDS diagnosis by WHO classification differed across groups. The stratification factor of baseline platelet counts was well matched.
Patient disposition. *Stratified by baseline platelet count ≥ or < 50 × 109/L.
Demographic and clinical characteristics
| Characteristic . | Placebo (n = 13) . | Romiplostim . | Total (n = 40) . | |
|---|---|---|---|---|
| 500 μg (n = 13) . | 750 μg (n = 14) . | |||
| Median age, y (range) | 64 (58-86) | 72 (56-86) | 72 (61-81) | 71 (56-86) |
| Sex, n (%) | ||||
| Female | 6 (46) | 3 (23) | 7 (50) | 16 (40) |
| ECOG PS, n (%) | ||||
| 0 | 9 (69) | 6 (46) | 5 (36) | 20 (50) |
| 1 | 4 (31) | 7 (54) | 9 (64) | 20 (50) |
| MDS diagnosis, n (%) | ||||
| RA | 2 (15) | 3 (23) | 0 (0) | 5 (13) |
| RARS | 2 (15) | 1 (8) | 0 (0) | 3 (8) |
| RAEB-1 | 3 (23) | 5 (39) | 2 (14) | 10 (25) |
| RAEB-2 | 0 (0) | 1 (8) | 1 (7) | 2 (5) |
| RCMD | 4 (31) | 2 (15) | 8 (57) | 14 (35) |
| RCMD-RS | 1 (8) | 0 (0) | 0 (0) | 1 (3) |
| MDS-U | 1 (8) | 1 (8) | 0 (0) | 2 (5) |
| MDS with isolated del 5Q | 0 (0) | 0 (0) | 2 (14) | 2 (5) |
| IPSS score, n (%) | ||||
| Low | 1 (8) | 1 (8) | 1 (7) | 3 (8) |
| Intermediate-1 | 5 (38) | 9 (69) | 9 (64) | 23 (58) |
| Intermediate-2 | 7 (54) | 3 (23) | 4 (29) | 14 (35) |
| Median duration of MDS, wk (interquartile range) | 41 (24, 144) | 35 (7, 109) | 47 (5, 86) | 43 (7, 124) |
| Bleeding events in previous year, n (%) | 4 (31) | 4 (31) | 5 (36) | 13 (33) |
| History of transfusions, n (%) | 10 (77) | 9 (69) | 11 (79) | 30 (75) |
| Median baseline ANC, 109/L (range) | 1.9 (0.1-9.0) | 1.1 (0.3-44.8) | 1.1 (0.2-3.7) | 1.2 (0.1-44.8) |
| Medain baseline platelets, < 50 × 109/L (range) | 25 (8-433) | 40 (7-296) | 41 (5-160) | 40 (5-433) |
| Baseline platelets, < 50 × 109/L, n (%) | 8 (62) | 8 (62) | 8 (57) | 24 (60) |
| Characteristic . | Placebo (n = 13) . | Romiplostim . | Total (n = 40) . | |
|---|---|---|---|---|
| 500 μg (n = 13) . | 750 μg (n = 14) . | |||
| Median age, y (range) | 64 (58-86) | 72 (56-86) | 72 (61-81) | 71 (56-86) |
| Sex, n (%) | ||||
| Female | 6 (46) | 3 (23) | 7 (50) | 16 (40) |
| ECOG PS, n (%) | ||||
| 0 | 9 (69) | 6 (46) | 5 (36) | 20 (50) |
| 1 | 4 (31) | 7 (54) | 9 (64) | 20 (50) |
| MDS diagnosis, n (%) | ||||
| RA | 2 (15) | 3 (23) | 0 (0) | 5 (13) |
| RARS | 2 (15) | 1 (8) | 0 (0) | 3 (8) |
| RAEB-1 | 3 (23) | 5 (39) | 2 (14) | 10 (25) |
| RAEB-2 | 0 (0) | 1 (8) | 1 (7) | 2 (5) |
| RCMD | 4 (31) | 2 (15) | 8 (57) | 14 (35) |
| RCMD-RS | 1 (8) | 0 (0) | 0 (0) | 1 (3) |
| MDS-U | 1 (8) | 1 (8) | 0 (0) | 2 (5) |
| MDS with isolated del 5Q | 0 (0) | 0 (0) | 2 (14) | 2 (5) |
| IPSS score, n (%) | ||||
| Low | 1 (8) | 1 (8) | 1 (7) | 3 (8) |
| Intermediate-1 | 5 (38) | 9 (69) | 9 (64) | 23 (58) |
| Intermediate-2 | 7 (54) | 3 (23) | 4 (29) | 14 (35) |
| Median duration of MDS, wk (interquartile range) | 41 (24, 144) | 35 (7, 109) | 47 (5, 86) | 43 (7, 124) |
| Bleeding events in previous year, n (%) | 4 (31) | 4 (31) | 5 (36) | 13 (33) |
| History of transfusions, n (%) | 10 (77) | 9 (69) | 11 (79) | 30 (75) |
| Median baseline ANC, 109/L (range) | 1.9 (0.1-9.0) | 1.1 (0.3-44.8) | 1.1 (0.2-3.7) | 1.2 (0.1-44.8) |
| Medain baseline platelets, < 50 × 109/L (range) | 25 (8-433) | 40 (7-296) | 41 (5-160) | 40 (5-433) |
| Baseline platelets, < 50 × 109/L, n (%) | 8 (62) | 8 (62) | 8 (57) | 24 (60) |
ECOG PS indicates Eastern Cooperative Oncology Group performance status; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RAEB-1, refractory anemia with excess blasts-1; RAEB-2, refractory anemia with excess blasts-2; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, refractory cytopenia with multilineage dysplasia and ringed sideroblasts; and MDS-U, myelodysplastic syndrome, unclassified.
Efficacy
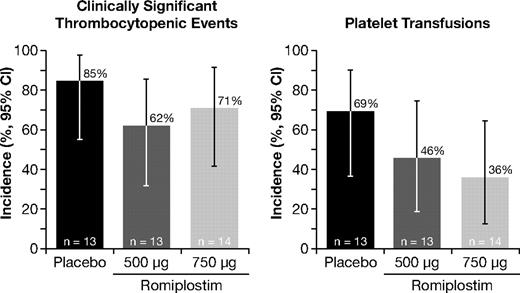
Eleven of 13 patients (85%) treated with placebo experienced CSTE compared with 8 of 13 patients (62%) in the romiplostim 500-μg group and 10 of 14 patients (71%) in the 750-μg group (Figure 2). The difference in CSTE rates between the romiplostim and placebo groups did not achieve statistical significance. The proportion of patients who experienced a CSTE was similar for those with IPSS low/intermediate-1 risk (18 of 26, 69%) and those with intermediate-2 risk (11 of 14, 76%) MDS. The difference in CSTE rates between the romiplostim and placebo groups was comparable among low/intermediate-1 risk and intermediate-2 risk patients (data not shown). Twenty-three of the 29 patients with CSTE had severe thrombocytopenia at baseline. When examined by treatment cycle, the incidence of severe thrombocytopenia (platelet count < 50 × 109/L or receipt of platelet transfusion during the cycle) was consistently lower in the romiplostim groups than the placebo group, with the greatest difference between romiplostim and placebo found in the 750-μg group (Table 2).
Effect of romiplostim on the incidence of clinically significant thrombocytopenic events (left panel) and platelet transfusions (right panel). CI indicstes confidence interval.
Effect of romiplostim on the incidence of clinically significant thrombocytopenic events (left panel) and platelet transfusions (right panel). CI indicstes confidence interval.
Patient incidence of severe thrombocytopenia* and platelet transfusions by azacitidine treatment cycle
| . | Incidence, n/N (%) . | Difference in incidence, % (95% CI) . | |||
|---|---|---|---|---|---|
| Placebo . | Romiplostim 500 μg . | Romiplostim 750 μg . | Romiplostim 500 μg vs placebo . | Romiplostim 750 μg vs placebo . | |
| Severe thrombocytopenia | |||||
| Any cycle | 11/13 (85) | 9/13 (69) | 10/14 (71) | −15.4 (−47.3 to 16.5) | −13.2 (−43.9 to 17.5) |
| Cycle 1 | 11/13 (85) | 9/13 (69) | 9/14 (64) | −15.4 (−47.3 to 16.5) | −20.3 (−52.2 to 11.6) |
| Cycle 2 | 7/11 (64) | 5/10 (50) | 5/13 (38) | −13.6 (−55.7 to 28.5) | −25.1 (−63.9 to 13.7) |
| Cycle 3 | 8/11 (73) | 6/10 (60) | 2/11 (18) | −12.7 (−52.9 to 27.5) | −54.5 (−89.3 to −19.7) |
| Cycle 4 | 5/10 (50) | 4/9 (44) | 3/10 (30) | −5.6 (−50.5 to 39.3) | −20.0 (−62.0 to 22.2 |
| Platelet transfusions | |||||
| Any cycle | 9/13 (69) | 6/13 (46) | 5/14 (36) | −23.1 (−60.0 to 13.9) | −33.5 (−69.0 to 2.0) |
| Cycle 1 | 9/13 (69) | 6/13 (46) | 5/14 (36) | −23.1 (−60.0 to 13.9) | −33.5 (−69.0 to 2.0) |
| Cycle 2 | 6/11 (55) | 4/10 (40) | 3/13 (23) | −14.5 (−56.8 to 27.7) | −31.5 (−68.8 to 5.8) |
| Cycle 3 | 5/11 (46) | 3/10 (30) | 2/11 (18) | −15.5 (−56.4 to 25.4) | −27.3 (−64.5 to 9.9) |
| Cycle 4 | 4/10 (40) | 3/9 (33) | 0/10 (0) | −6.7 (−49.9 to 36.6) | −40.0 (−70.4 to −9.6) |
| . | Incidence, n/N (%) . | Difference in incidence, % (95% CI) . | |||
|---|---|---|---|---|---|
| Placebo . | Romiplostim 500 μg . | Romiplostim 750 μg . | Romiplostim 500 μg vs placebo . | Romiplostim 750 μg vs placebo . | |
| Severe thrombocytopenia | |||||
| Any cycle | 11/13 (85) | 9/13 (69) | 10/14 (71) | −15.4 (−47.3 to 16.5) | −13.2 (−43.9 to 17.5) |
| Cycle 1 | 11/13 (85) | 9/13 (69) | 9/14 (64) | −15.4 (−47.3 to 16.5) | −20.3 (−52.2 to 11.6) |
| Cycle 2 | 7/11 (64) | 5/10 (50) | 5/13 (38) | −13.6 (−55.7 to 28.5) | −25.1 (−63.9 to 13.7) |
| Cycle 3 | 8/11 (73) | 6/10 (60) | 2/11 (18) | −12.7 (−52.9 to 27.5) | −54.5 (−89.3 to −19.7) |
| Cycle 4 | 5/10 (50) | 4/9 (44) | 3/10 (30) | −5.6 (−50.5 to 39.3) | −20.0 (−62.0 to 22.2 |
| Platelet transfusions | |||||
| Any cycle | 9/13 (69) | 6/13 (46) | 5/14 (36) | −23.1 (−60.0 to 13.9) | −33.5 (−69.0 to 2.0) |
| Cycle 1 | 9/13 (69) | 6/13 (46) | 5/14 (36) | −23.1 (−60.0 to 13.9) | −33.5 (−69.0 to 2.0) |
| Cycle 2 | 6/11 (55) | 4/10 (40) | 3/13 (23) | −14.5 (−56.8 to 27.7) | −31.5 (−68.8 to 5.8) |
| Cycle 3 | 5/11 (46) | 3/10 (30) | 2/11 (18) | −15.5 (−56.4 to 25.4) | −27.3 (−64.5 to 9.9) |
| Cycle 4 | 4/10 (40) | 3/9 (33) | 0/10 (0) | −6.7 (−49.9 to 36.6) | −40.0 (−70.4 to −9.6) |
Severe thrombocytopenia: platelet count < 50 × 109/L or receipt of platelet transfusion during the cycle.
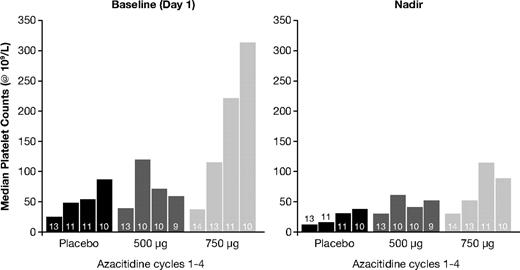
The proportion of patients receiving platelet transfusions was 69% in the placebo group, 46% with romiplostim 500 μg, and 36% with romiplostim 750 μg (Figure 2). The difference in platelet transfusion rates was not statistically significant (Table 2). The proportion of patients who received a platelet transfusion in those with IPSS low/intermediate-1 risk was 38% (10 of 26) and in those with intermediate-2 risk was 86% (12 of 14). The difference in platelet transfusion rates between the romiplostim and placebo groups was lower in low/intermediate-1 risk than intermediate-2 risk patients and followed a pattern similar to the overall patient population (data not shown). Thirty-four transfusions were administered in the romiplostim 750-μg group compared with 79 and 105 transfusions in the placebo and romiplostim 500-μg groups, respectively. Patients in the romiplostim 500-μg and 750-μg groups received a median of 0 units (range, 0-72 units) and 0 units (range, 0-29 units), respectively, compared with 6 units (range, 0-38 units) in the placebo group. The proportion of patients receiving platelet transfusions tended to decrease, particularly in the romiplostim 750-μg group, where the incidence declined from 36% in the first cycle to 0% in the fourth cycle (Table 2). In comparison, the incidence of platelet transfusions in the placebo group ranged from 69% in cycle 1 to 40% in cycle 4. When examined by treatment cycle, the receipt of platelet transfusions was consistently lower in the romiplostim groups than the placebo group, with the greatest difference between romiplostim and placebo found in the 750-μg group (Table 2).
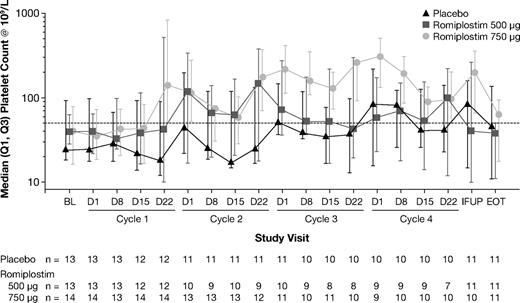
Romiplostim 750 μg increased median platelet counts with effects evident by the end of the first cycle and maintained until the end-of-treatment visit (Figure 3). In comparison, romiplostim 500 μg raised median platelet counts during the second treatment cycle, but this effect was not maintained in the third or fourth cycles. Romiplostim 750 μg significantly raised median platelet counts on day 1 of cycle 3 (nonparametric P = .0373) and at the nadir of cycle 3 (P = .0035) compared with placebo (Figure 4). The platelet response (hematologic improvement in platelets) incidence after 4 cycles was 56% (5 of 9), 50% (5 of 10), and 89% (8 of 9) in the placebo, romiplostim 500-μg, and 750-μg groups, respectively.
Effect of romiplostim on median platelet counts on day 1 of each treatment cycle (left panel) and on median platelet counts at nadir during each treatment cycle (right panel). P values in a nonparametric test comparing median platelet counts between the romiplostim 500-μg and placebo groups were 0.4233, 0.8389, 0.8389, and 0.8137 on day 1 of cycles 1, 2, 3, and 4, respectively, and 0.4986, 0.2905, 0.2905, and 0.5093 at the nadir of cycles 1, 2, 3, and 4, respectively. P values comparing median platelet counts between the romiplostim 750-μg and placebo groups were 0.8445, 0.2289, 0.0373, and 0.1198 on day 1 of cycles 1, 2, 3, and 4, respectively, and 0.3408, 0.2289, 0.0035, and 0.3833 at the nadir of cycles 1, 2, 3, and 4, respectively.
Effect of romiplostim on median platelet counts on day 1 of each treatment cycle (left panel) and on median platelet counts at nadir during each treatment cycle (right panel). P values in a nonparametric test comparing median platelet counts between the romiplostim 500-μg and placebo groups were 0.4233, 0.8389, 0.8389, and 0.8137 on day 1 of cycles 1, 2, 3, and 4, respectively, and 0.4986, 0.2905, 0.2905, and 0.5093 at the nadir of cycles 1, 2, 3, and 4, respectively. P values comparing median platelet counts between the romiplostim 750-μg and placebo groups were 0.8445, 0.2289, 0.0373, and 0.1198 on day 1 of cycles 1, 2, 3, and 4, respectively, and 0.3408, 0.2289, 0.0035, and 0.3833 at the nadir of cycles 1, 2, 3, and 4, respectively.
The dose of azacitidine was delayed because of thrombocytopenia in 4 patients: 1 in the placebo group, 2 in the romiplostim 500-μg group, and 1 in the romiplostim 750-μg group. Similarly, the incidence of MDS treatment responses to azacitidine did not appear affected by concomitant romiplostim treatment. The response rates were 15% (2 of 13), 8% (1 of 13), and 14% (2 of 14) in the placebo, romiplostim 500-μg, and 750-μg groups, respectively, and included 4 complete responses and 1 partial response. Patients who responded to azacitidine had similar CSTE (67% vs 79%) and transfusion (33% vs 50%) rates and similar mean platelet nadirs (32 vs 28 × 109/L) as nonresponders to azacitidine. For each parameter, the 95% confidence interval of the difference between responders and nonresponders included zero.
Safety
The incidence of most adverse events was well balanced across groups (Table 3), although several types of events were reported more frequently with romiplostim, including diarrhea, pain in extremity, dizziness, hypotension, and anorexia. Treatment-related adverse events were reported more frequently with romiplostim than placebo (52% vs 31%), but all were grade 1 or 2, except for grade 3 arthralgia in 1 patient in the romiplostim 500-μg group and 1 patient with grade 3 rash in the 750-μg group. Three patients had treatment-related serious adverse events: 2 in the placebo group (myelofibrosis and erythema nodosum) and the aforementioned patient with grade 3 rash in the romiplostim 750-μg group. Two patients died during this study, both in the placebo group, 1 from fungal pneumonia and 1 from endocarditis.
Safety
| Characteristic . | Placebo (n = 13), n (%) . | Romiplostim . | ||
|---|---|---|---|---|
| 500 μg (n = 13), n (%) . | 750 μg (n = 14), n (%) . | Total (n = 27), n (%) . | ||
| Patients with adverse events | 13 (100) | 13 (100) | 14 (100) | 27 (100) |
| Hematologic adverse events* | ||||
| Anemia | 3 (23) | 1 (8) | 4 (29) | 5 (19) |
| Neutropenia | 4 (31) | 5 (38) | 0 (0) | 5 (19) |
| Febrile neutropenia | 6 (46) | 2 (15) | 1 (7) | 3 (11) |
| Thrombocytopenia | 4 (31) | 1 (8) | 1 (7) | 2 (7) |
| Nonhematologic adverse events* | ||||
| Constipation | 5 (38) | 7 (54) | 7 (50) | 14 (52) |
| Nausea | 7 (54) | 5 (39) | 6 (43) | 11 (41) |
| Diarrhea | 1 (8) | 4 (31) | 6 (43) | 10 (37) |
| Fatigue | 5 (38) | 4 (31) | 3 (21) | 7 (26) |
| Pain in extremity | 1 (8) | 2 (15) | 5 (36) | 7 (26) |
| Dizziness | 1 (8) | 2 (15) | 4 (29) | 6 (22) |
| Hypotension | 1 (8) | 1 (8) | 6 (43) | 7 (26) |
| Dyspnea | 6 (46) | 4 (31) | 2 (14) | 6 (22) |
| Peripheral edema | 4 (31) | 2 (15) | 4 (29) | 6 (22) |
| Contusion | 3 (23) | 4 (31) | 3 (21) | 7 (26) |
| Rash | 2 (15) | 2 (15) | 4 (29) | 6 (22) |
| Anorexia | 0 (0) | 2 (15) | 4 (29) | 6 (22) |
| Patients with serious adverse events | 10 (77) | 6 (46) | 10 (71) | 16 (59) |
| Patients with treatment-related serious adverse events | 2 (15) | 0 (0) | 1 (7) | 2 (7) |
| Myelofibrosis | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Erythema nodosum | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Rash and hypersensitivity | 0 (0) | 0 (0) | 1 (7) | 1 (4) |
| Bleeding events | 7 (54) | 8 (62) | 7 (50) | 15 (56) |
| Grade ≥ 3 bleeding events | 2 (15) | 1 (8) | 0 (0) | 1 (4) |
| Pulmonary hemorrhage | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Hematoma | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Epistaxis | 0 (0) | 1 (8) | 0 (0) | 1 (4) |
| Deaths | 2 (15) | 0 (0) | 0 (0) | 0 (0) |
| Endocarditis | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Fungal pneumonia | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Characteristic . | Placebo (n = 13), n (%) . | Romiplostim . | ||
|---|---|---|---|---|
| 500 μg (n = 13), n (%) . | 750 μg (n = 14), n (%) . | Total (n = 27), n (%) . | ||
| Patients with adverse events | 13 (100) | 13 (100) | 14 (100) | 27 (100) |
| Hematologic adverse events* | ||||
| Anemia | 3 (23) | 1 (8) | 4 (29) | 5 (19) |
| Neutropenia | 4 (31) | 5 (38) | 0 (0) | 5 (19) |
| Febrile neutropenia | 6 (46) | 2 (15) | 1 (7) | 3 (11) |
| Thrombocytopenia | 4 (31) | 1 (8) | 1 (7) | 2 (7) |
| Nonhematologic adverse events* | ||||
| Constipation | 5 (38) | 7 (54) | 7 (50) | 14 (52) |
| Nausea | 7 (54) | 5 (39) | 6 (43) | 11 (41) |
| Diarrhea | 1 (8) | 4 (31) | 6 (43) | 10 (37) |
| Fatigue | 5 (38) | 4 (31) | 3 (21) | 7 (26) |
| Pain in extremity | 1 (8) | 2 (15) | 5 (36) | 7 (26) |
| Dizziness | 1 (8) | 2 (15) | 4 (29) | 6 (22) |
| Hypotension | 1 (8) | 1 (8) | 6 (43) | 7 (26) |
| Dyspnea | 6 (46) | 4 (31) | 2 (14) | 6 (22) |
| Peripheral edema | 4 (31) | 2 (15) | 4 (29) | 6 (22) |
| Contusion | 3 (23) | 4 (31) | 3 (21) | 7 (26) |
| Rash | 2 (15) | 2 (15) | 4 (29) | 6 (22) |
| Anorexia | 0 (0) | 2 (15) | 4 (29) | 6 (22) |
| Patients with serious adverse events | 10 (77) | 6 (46) | 10 (71) | 16 (59) |
| Patients with treatment-related serious adverse events | 2 (15) | 0 (0) | 1 (7) | 2 (7) |
| Myelofibrosis | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Erythema nodosum | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Rash and hypersensitivity | 0 (0) | 0 (0) | 1 (7) | 1 (4) |
| Bleeding events | 7 (54) | 8 (62) | 7 (50) | 15 (56) |
| Grade ≥ 3 bleeding events | 2 (15) | 1 (8) | 0 (0) | 1 (4) |
| Pulmonary hemorrhage | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Hematoma | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Epistaxis | 0 (0) | 1 (8) | 0 (0) | 1 (4) |
| Deaths | 2 (15) | 0 (0) | 0 (0) | 0 (0) |
| Endocarditis | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Fungal pneumonia | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
Adverse events reported in ≥ 20% of romiplostim-treated patients or ≥ 30% of placebo-treated patients.
Three cases of AML progression occurred, 1 in the placebo group and 2 in the romiplostim 500-μg group. The placebo patient was female, 84 years old, had been diagnosed with MDS 33 months before entering the study, and had a WHO classification of refractory anemia with excess blasts-1 and an IPSS score of 1.5 at baseline with an entry platelet count of 19 × 109/L. After 15 weeks, she discontinued treatment and was diagnosed with progression to AML. The patient had erythroleukemia and the end of treatment biopsy showed 26% blasts of nonerythroid cells. The first romiplostim patient was 74 years old, had been diagnosed with MDS 31 months before receiving romiplostim, and had a WHO classification of refractory anemia with excess blasts-1 and an IPSS score of 1.0 at baseline with 5% to 10% blasts. He was previously treated with lenalidomide and had a platelet count of 33 × 109/L at study entry. Romiplostim 500 μg was administered for 11 weeks and then discontinued after development of pancytopenia, considered possibly related to azacitidine. Biopsies taken after 13 and 15 weeks on study showed 64% and 54% bone marrow blasts, respectively, leading to the diagnosis of AML. The second romiplostim patient was 78 years old, had been diagnosed with MDS 1 month before receiving romiplostim, and had a WHO classification of refractory anemia with excess blasts-1 and an IPSS score of 2.0 at baseline with 5% to 10% blasts. Her platelet count before starting therapy was 6 × 109/L. Romiplostim 500 μg was administered for 18 weeks and treatment was discontinued because of disease progression to AML based on bone marrow blast cells ≥ 20%. Cytogenetics were unchanged from baseline in both cases. Bone marrow blasts ≥ 20% were not detected in any other patient.
Of 27 patients for whom both pretreatment and end-of-treatment reticulin-stained bone marrow biopsies were available, the reticulin grade was increased in 13 (3 in placebo, 4 in 500 μg romiplostim, 6 in 750 μg romiplostim) unchanged in 12 (4 in placebo, 6 in 500 μg romiplostim, 2 in 750 μg romiplostim), and decreased in 2 (1 in placebo, 1 in 500 μg romiplostim). Of the 13 patients in whom the reticulin grade was increased, the end-of-treatment biopsy showed grade 1 in 6 patients, grade 2 in 3 patients, and grade 3 in 4 patients. No grade 4 reticulin was observed and trichrome staining was absent in each case.
The overall incidence of bleeding events did not differ across groups (Table 3); most bleeding events were rated grade 1 or 2. Bleeding events occurred more frequently in patients with baseline platelet counts < 50 × 109/L (75% of patients in placebo and romiplostim arms) than those with higher baseline platelet counts (20% and 27% with placebo and romiplostim, respectively). Clinically relevant grade ≥ 3 bleeding events occurred in 2 patients (15%) in the placebo group (pulmonary hemorrhage on day 40 and hematoma on day 105) and in 1 patient (4%) on romiplostim (epistaxis on day 98 in the 500-μg group).
Discussion
The results of this study suggest that romiplostim may provide important clinical benefits in patients with low- and intermediate-risk MDS receiving azacitidine, which are most apparent with the weekly 750-μg dose. This is supported by the following findings. First, romiplostim reduced the incidence of CSTE, although this reduction was not statistically significant. Second, romiplostim increased median platelet counts over time, reaching ≥ 100 × 109/L at most weekly assessments during the third and fourth cycles. This improvement was associated with significantly higher platelet counts at the start of cycle 3 as well as a significantly higher platelet count nadir during the same cycle compared with placebo. Third, there was a reduction in the incidence and number of platelet transfusions in the romiplostim 750-μg group compared with placebo, although this difference was not statistically significant. Finally, severe bleeding events occurred in 2 patients treated with placebo, 1 treated with 500 μg of romiplostim, and none treated with 750 μg of romiplostim.
The safety profile of romiplostim was generally similar to placebo when administered in combination with azacitidine. The overall incidence of adverse events was generally comparable between groups, although some treatment-related adverse events were more common with romiplostim than with placebo. However, nearly all such events were mild or moderate (grade 1 or 2). Two patients treated with romiplostim had grade 3 treatment-related adverse events, and 1 of these was the only patient with a treatment-related serious adverse event in the romiplostim groups.
A previous study using romiplostim as a single agent in patients with lower-risk MDS showed transient blast increases in 9% of patients and progression to AML in 5% of patients.20 Three patients developed AML in the current study: 1 treated with placebo and 2 treated with romiplostim. Two patients were intermediate-1 risk and the other was intermediate-2 risk at study entry. The time from diagnosis of MDS to AML progression was 37 months for the placebo-treated patient and 34 and 22 months for the romiplostim-treated patients. Approximately 25% of intermediate-1 and intermediate-2 risk MDS patients progress to AML after a median of 3.3 and 1.1 years, respectively (2.2 and 1.4 years, respectively, for the subset > 70 years of age).6 Moreover, the platelet count is inversely correlated with the time to AML progression in MDS: the median time to AML is 1.3 years for patients with platelet counts of 20 to 49 × 109/L and 3.8 years for patients with platelet counts > 100 × 109/L.10 Therefore, progression of the placebo- and romiplostim-treated patients in this study seems consistent with the expected disease course for severely thrombocytopenic patients with IPSS risk scores of intermediate-1 and intermediate-2. Nevertheless, because of the small patient cohort and relatively short follow-up time, the present study cannot determine whether romiplostim influences risk of AML transformation. An ongoing open-label extension study is evaluating the long-term safety and efficacy of romiplostim in patients with MDS and thrombocytopenia.
This study has several limitations. First, the study had a relatively small sample size with only 13 to 14 patients per arm. Patients were stratified by baseline platelet counts in an effort to balance groups for CSTE risk, but the small sample size led to some imbalances in baseline characteristics. Notably, a higher proportion of patients had intermediate-2 risk in the placebo group; however, a post hoc analysis demonstrated that efficacy effects in the higher-risk group were consistent with those in the overall population. Second, CSTE was defined as the primary endpoint and included grade 3 or 4 thrombocytopenia events starting at day 15. This endpoint may not document the potential clinical benefit of romiplostim because platelet responses did not occur in most patients until day 22. Third, because fixed-dose levels were evaluated, the dose of romiplostim was not titrated in each patient to bring platelet counts into a desired range. As a result, the potential clinical benefit of romiplostim may have been underestimated. Fourth, the assessment of treatment response after 4 cycles of azacitidine may not reflect the true response rate because some responses may become evident after multiple additional cycles only. Finally, the platelet count increases and reduced platelet transfusions experienced by some patients may have been the result of responses to azacitidine rather than romiplostim, particularly during later cycles. However, both placebo and romiplostim patients received azacitidine therapy, and patients who responded to azacitidine had similar CSTE and transfusion rates, and similar mean platelet nadirs, as nonresponders to azacitidine.
In conclusion, this trial suggests that romiplostim may provide important clinical benefits in patients with MDS receiving azacitidine, including reductions in platelet transfusions and severe bleeding. The 750-μg dose appears to be more effective than the 500-μg dose in raising platelet counts and reducing transfusions. These findings support further investigation of romiplostim in ameliorating treatment-induced thrombocytopenia in MDS. Additional phase 2 studies are evaluating romiplostim in patients with MDS treated with either decitabine or lenalidomide. The clinical benefit of romiplostim treatment will be further explored in a randomized, double-blind, placebo-controlled single-agent study, which will assess the incidence of clinically significant bleeding, overall survival, and progression to AML in patients with low-risk MDS.
Presented in part at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Barry M. Weichman and PAREXEL who provided scientific writing assistance.
This work was supported by Amgen. J.L.G. was supported by Amgen (grant K24CA100287).
Authorship
Contribution: H.M.K., F.J.G., P.L.G., and D.P.B. conceived and designed the study; H.M.K., F.J.G., P.L.G., R.L.P., E.S.W., J.L.G., and G.G.-M. provided study materials or patients; K.H. collected and assembled the data; H.M.K., P.L.G., K.H., J.L.F., and D.P.B. analyzed and interpreted data; H.M.K. and D.P.B. wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: D.P.B., J.L.F., and K.H. held employment or a leadership position. P.L.G. was a consultant or had an advisory role. H.M.K., F.J.G., and P.L.G. obtained research funding. The remaining authors declare no competing financial interests.
Correspondence: Hagop M. Kantarjian, Leukemia Department, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: hkantarj@mdanderson.org.